Introduction
Wuhan city in the Hubei province of China experienced an increase in the number of pneumonia cases of unknown aetiology in the month of December 2019. The incidence was shared with the World Health Organisation China country office on 31st December 2019.1 A virus not like anything seen before related to Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory syndrome (SARS) was arraigned. Although initially in the first week of January it was estimated to affect only few cluster of patients in China the scientific community was expecting the possibility of further human to human transmission and the global spread due to international travel of undetected and asymptomatic cases during the incubation period.
Setting and design
The study is cumulative of the recent literature released by the WHO and Chinese scientists handling the samples and Interim measures on controlling the 2019n-Corona virus and by American National Institute of Health and World Health Organization.
Exclusion criteria
An article with no proves and dated before 2000. The study was conducted at GIMS-VRDL, Department of Microbiology, Gulbarga Institute of Medical Sciences, Kalaburagi, Karnataka and studies were selected using PRISMA 2009 checklist.
Data items
We searched through Google, PubMed, Pub Med Central, CAS, Citebase, DOAJ, Embase, Embiology, MEDLINE, OAIster, SCImago, SCOPUS, SOCOLAR and Zetoc by using the keywords.
Spread of the novel virus
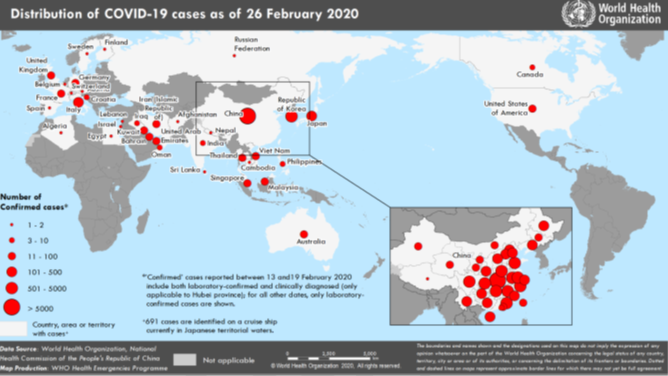
Within a period of 57 days the COVID-19 has affected more than 82138 cases globally and has caused more than 2744 deaths in mainland China, followed by Iran 19, South Korea 13 and Italy 12 according to WHO situation reports and Chinese Authorities.2 2 A history of travel to China or contact with people arriving from China and affected countries like Japan, Vietnam Korea, Thailand and United states of America3 and 20 more countries with confirmed cases having fever, difficulty in breathing and severe coughing before 1st of January or 15 days from onset of symptom have been considered as suspected cases for Wuhan Corona virus. The incubation period of COVID-19 is 0-14 days.4, 5, 6 11-14 According to a report released by WHO on 29th January 2020, the 2019n-corona virus now exceeds the number of SARS affected individuals globally.2 2 According to the situation report by WHO released on 19th February 2020 COVID-19 has spread to 25 countries. Within China the Case Fatality Ratio (CFR) is 2.3% and the infection fatality ratio IFR ranges between 0.3% and 1%.
In recent times two highly pathogenic corona virus namely MERS and SARS caused mortality and morbidity globally on a large scale. Both the virus had animals as common reservoir and mode of spread was air droplets. 2019n-corona virus is no different from its virulent cousins. The first case was detected from consumption of wild animal meat brought from the wet seafood market in Wuhan.7 Recent reports suggest bats as a potential carrier for the novel virus. With a high efficacy mutation rate effects of 2019n-corona virus, infection and its extent of spread is uncertain. Scientists believe that the numbers are underestimated as more number of carriers would have been exposed other than the sea food market in Wuhan city. The complete city has been put on a lock down with the Chinese officials announcing public holiday and restriction on unnecessary travel and transport within and outside the country.
Human Corona viruses (HCoV) are known to cause disease in mammals and birds. It is a single stranded enveloped RNA virus that is spherical or pleomorphic with glycoprotein projections. With four subtypes namely Alpha, Beta, Gamma and Delta, HCoV has 7 serotypes. The mode of transmission is airborne through zoonotic droplets, and the viral replication occurs in the ciliated epithelium.8 Once the glycoprotein attaches the ciliated epithelium they penetrate the cells and replicate in a span of 3-4 days and cause cellular damage and inflammatory reactions at the site of infection.9
In 2002 Guangdong province of china, similar severe type of atypical pneumonia caused global concern with more than 12 countries affected by the viral infection due to international travel.10 Later this subtype Beta Corona virus was named as SARS. In 2012 MERS emerged as a potentially pathogenic corona virus and caused an astounding 774 deaths and more than 8000 affected cases in Saudi Arabia, Africa, European countries and America.11
Phylogenetic analysis so far
0n 29th January 2020, Roujian Lu et al from Chinese Academy of Science collected bronchoalveolar lavage sample from 10 positive cases from Huanan sea food market and performed Sanger genomic sequencing to determine the full length of COVID-19 phylogenetic analysis. They have found that the 2019n-corona virus is closely related to bat-SL-CoVZC45 and batSL-CoVZXC21 of SARS like corona virus (88% identity) but since the subtype was found to be Sarbecovirus of the genus Beta coronavirus, hence can be considered a new human infecting betacorona virus.12 The report also revealed that 2019n-corona virus has a similar receptor binding domain structure as that of SARS-CoV and that it has the capability of binding to the angiotensin converting enzyme receptor in humans.
Indian perspective
The Central Government of India through the Department of Health and Research (DHR) and Indian Council of Medical Research (ICMR)has taken immediate steps as part of the preparedness of Viral Research and Diagnostic Laboratories across the country for dealing with the COVID-19. The Ministry of Health and Family Welfare made literature available regarding the clinical management, surveillance and sample collection from suspected cases. All cases with Severe Acute Respiratory Illness (SARI) with a history of fever, coughing and difficulty in breathing requiring hospitalization with no other aetiology explaining the clinical presentation are to be considered a potential case. Added to the fact a history of travel to Wuhan city of China or affected countries by the patient or his/her relatives in contact within 14 days prior to symptoms are to be screened with precaution. Health care workers in direct contact with treating severe acute respiratory illness cases also need to take necessary precautions as advised by the Department of Health and Research. Use of Personal Protective Equipment (PPE) including head cap, N95 masks, eye wear, face shields, full sleeve high collar apron, gowns, double gloves, and shoe covers are mandatory while treating or during sample collection of suspected cases. On 19th January 2020 The Department of Health and Research, New Delhi released the form for specimen collection and transport to National Institute of Virology Pune and suggested to adhere to WHO Interim guidelines for preventive steps during sample collection and transport. Specimen type for 2019n Corona virus include nasopharyngeal / oropharyngeal swab, bronchoalveolar lavage, tracheal/nasopharyngeal aspirate, sputum, serum and tissue biopsy/ autopsy from lungs. The sample need to be transported in Viral Transport medium maintaining the temperature at 4 degree Celsius if sent within 48 hours and -70 degree Celsius if sent within 5 days.
Diagram 2
Epidemic curve of COVID-19 outside China showing date of onset of symptom and likely exposure locations.13
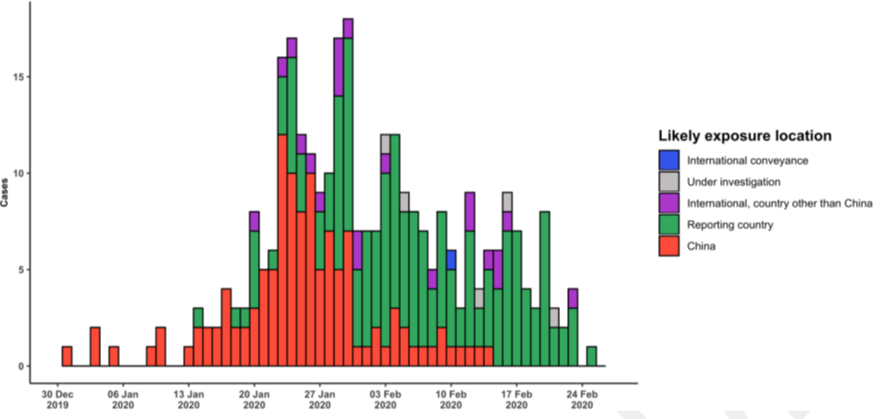
Diagram 4
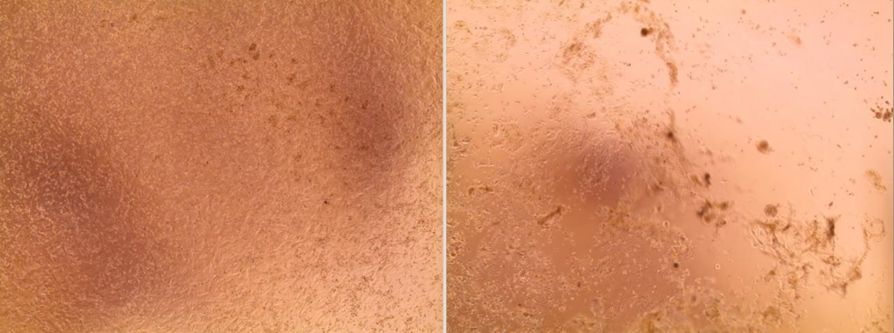
On the left, a cell layer not damaged by the viruses. On the right, a cell layer with a visible cytopathic effect (CPE); the cells infected by the virus have been destroyed.15
Estimating the spread
On 31st January WHO announced 2019n-corona virus as an global epidemic. With increasing number of cases each day passing the awareness and preparedness for the health care worker and concerned nursing staff is very important to avoid further spread of the viral infection. There have been efforts to estimate the number of cases affected by the COVID-19. Natsuko Imai Anne et al have suggested a formula to calculate the estimated number of cases including undetected to date.12
The formula takes into account the following entities:
C (Total number of cases), NO (number of cases detected overseas), P1 (daily probability of international travel), IT (daily probability of international travel), MT(mean time to detect a case), OW(daily outbound international travellers from Wuhan), CW(catchment population of Wuhan airport), MT(mean time to detection) IP (incubation period), OS (mean time from onset of symptoms to detection)
Summary
The main aim of the Central Government of India is to prevent further spread of the COVID-19 through promotion of respiratory hygiene. Department of Health Research has instructed all physicians that all suspected cases need to be isolated in separate wards and if placed in the same ward kept at a distance of 1meter. All health care workers attending the patients need to well trained and wear Personal Protective equipment at all times. Record of the health care personal entering the ward need to be maintained and the number of people visiting the ward need to be minimized to the lowest. The relatives accompanying the patient also need to be screened. Hospitals need to take care of environmental cleaning and regular disinfection of surfaces in contact with suspected COVID-19 cases by use of sodium hypochlorite and 70 per cent alcohol. The specimen best suited for detection as described by ICMR is oropharyngeal swab, nasopharyngeal swab and bronchoalveolar lavage (if possible), should be collected in viral transport media supplied by National Institute of Virology Pune and maintaining in a cold chain of 4 degrees if sent within 48 hours and -70 degree Celsius if sent within 5 days to NIV Pune for molecular detection through Real Time Polymerase chain reaction. With the current knowledge in perspective and no specific drugs or vaccinations recommended by WHO, physicians and research scientists from Chinese authorities found cases responding well to anti HIV drugs like Lopinavir/Nelfinavirin combination with Ribavir. National Institute of Health USA is working closely with vaccine developer companies in Wuhan using genetic sequences of the 2019n-CoV/ COVID-19. Further knowledge on genomic sequencing is necessary for developing diagnostic kits and vaccinations for the 2019n-CoV. Also reporting of cases and their management needs to be shared with researchers to be able to contribute, in control measures via an explicit line list and thorough history and management of such cases. Research on COVID-19 will focus on serological analysis, development of specific treatments, vaccination and viral pathogenesis.
Financial support and sponsorship
The research has been funded by the Department of Health Research and Indian Council of Medical Research Government of India, New Delhi under the Viral Research and Diagnostic Laboratories scheme set up at the Department of Microbiology, Gulbarga Institute of Medical Sciences, Kalaburagi, Karnataka, India.
Acknowledgement
This work was supported and funded by DHR-ICMR Govt of India, New Delhi under the Viral Research and Diagnostic Laboratory scheme. We would like to thank Dr Kavita Patil Director GIMS, Kalaburagi for providing necessary infrastructure and facilities to conduct the study. We would also like to thank Dr Umesh SR Principal GIMS, Kalaburagi for his guidance. We would like to thank Dr Sidhartha Giri Scientist E, DHR-ICMR New Delhi for an excellent technical coordination for the VRDL project.