Introduction
Superficial mycoses have been found in the last few decades to affect 20–25% of the world’s population, making them the second most common skin disease in the adult population.1, 2 Dermatophytes are fungi that invade and multiply within keratinized tissues (skin, hair, and nails) causing infection.3 The severity of the disease depends on the strain or the species of the dermatophyte and the susceptibility of the host to that fungus.4 Although dermatophytosis does not produce mortality, it does cause morbidity and poses a major public health problem, especially in tropical countries like India due to the hot and humid climate.5
The present study was undertaken to isolate and identify various fungal agents causing mycoses as detection of these agents becomes mandatory for the effective management of mycoses. In this study, we also evaluated the efficacy of seven antifungal medications using CLSI broth microdilution method. Establishment of a reference susceptibility testing method may allow the microbiologist to select the appropriate antifungal drug and help the clinician regarding the treatment of infections caused by dermatophytic fungi.
Materials and Methods
The present study of dermatophytosis was carried out in the Department of Microbiology, Sree Gokulam Medical College and Research Foundation, Venjaramoodu, over a period of one year from January 2016 to December 2016.
A total of 125 clinically diagnosed cases of dermatophytosis in all age groups and of both sexes, attending the outpatient department of Dermatology and Venereology at Sree Gokulam Medical College and Research Foundation were taken for this study.
Ethical approval
Ethics approval was obtained from Sree Gokulam Medical College and Research Foundation ethics committee SGMC-IEC-No-19/186/12/2015.
Sample collection and processing
A small portion of skin or nail scrapings, nail fragments or hair roots was placed on a clean glass microscopic slide. A drop of 10% KOH was added and examined for the presence of hyphae and arthrospores after 10-20 minutes. The rest of the specimen was cultured in sabouraud’s dextrose agar with chloramphenicol and cycloheximide (actidione). Each sample was inoculated into a pair of tubes. One tube was incubated at room temperature (25-300C) and the other at 370C. If growth was obtained on Sabourauds Dextrose agar, identification was made based on colony morphology, rate of growth, pigment production, microscopic appearance and other relevant tests
Potassium hydroxide mounts
It is prepared from the following ingredients:
Potassium hydroxide- 10gm
Glycerol- 10ml
Distilled water- 80ml
Composition of SDA with Cycloheximide and Chloramphenicol
Dextrose- 40gm
Peptone- 10gm
Agar- 20gm
Chloramphenicol- 0.05mg/ml dissolved in 95% ethanol.
Cycloheximide- 0.5 mg/ml dissolved in acetone.
Distilled water- 1000 ml.
Final pH- 5.4
The Lactophenol Cotton Blue (LCB) mount is used to study morphological features of fungal isolates.
Plain LCB
It contains following ingredients:
Melted phenol- 20ml
Lactic Acid- 20ml
Glycerol- 40ml
Cotton Blue- 0.05gm
Distilled water- 20ml
Antifungal susceptibility testing for dermatophytes
Broth microdilution method 6
Broth microdilution method
Requirements
Antifungal drugs.
RPMI-1640 medium with L-glutamine without sodium bicarbonate.
MOPS (3-morpholinopropane-1-sulfonic acid) buffer.
Sterile distilled water.
Dimethyl sulfoxide (DMSO).
Sabourauds dextrose agar.
Oat meal agar.
Sterile test tubes for drug dilution / inoculum preparation
Sterile disposable microtitre plates.
Sterile Micropipette / sterile tips / Gloves / disposable face masks.
Multichannel pipette.
Medium preparation
Dissolve 10.4 gram RPMI-1640 powder and 34.5 gram MOPS buffer in 900 ml sterile distilled water. Adjust pH to 7.0 using 4M NaOH. Make up to 1 litre with sterile distilled water. Filter sterilize using 0.22 μ filter. Check sterility and store at 40C.
Antifungal agent stock preparation
Antifungal stock solutions are prepared at concentration hundred times more than the highest concentration tested.10 ml stock solution was prepared for each drug.
Weight (mg) = volume (mL) × desired concentration (mg/mL) Antifungal potency
Weigh the appropriate amount of drug required for 10 ml stock.
Dissolve the drug in 10 ml DMSO (assuming 100% potency).
Aliquot into 5 × 2 ml snap cap tubes and store at -700Cuntil use.
The broth dilution test is performed by using sterile, disposable, multiwall microdilution plates (96 U-shaped wells). To prepare 200 μl of antifungal agent first pipette, 100 μl of RPMI 1640 medium is added to columns 2 to 9. For Fluconazole, 196 μl of RPMI 1640 is added to column 1. Then 4 μl of drug is dispensed into column 1. Serial dilutions are done using multichannel pipette and discard 100 μl from the column 9. Now columns 1 to 9 contains 100 μl solution. Final concentration after inoculation is {128, 64, 32, 16, 8, 4, 2, 1, 0.5}
Inoculum preparation
Dermatophytes are grown on oat meal agar slants until sufficient conidia are present (7-10) days. These oat meal agar slants are incubated at 300 C Overlay agar slant with 3-4 ml sterile distilled water. Gently scrape the surface of the slant with a fungal loop to obtain a conidial suspension. Allow slant to stand for 15-20 min for heavy, hyphal fragments and conidia to settle down. Without disturbing gently transfer the conidial suspension to a fresh tube. Adjust to 0.11 O.D. at 530 nm, and diluted 1:50 in the RPMI media to achieve double concentrated inoculum suspensions of 0.5 × 104 -4.0 × 10 4 CFU/ml.
Test procedure
Test was performed in sterile microtitre plates. Aliquots of 100 μl of drug dilutions were already inoculated in columns 1 to 9 microtitre wells. To this add 100 μl of inoculum suspensions to columns 1 to 9. Column 10 is filled with 200 μl of RPMI 1640 medium without drug or inoculum suspension, to serve as sterility control. Column 11 contains 100 μl of the inoculum and 100 μl of drug free RPMI 1640 medium to serve as growth controls.
Incubation
The microtitre plates were incubated at 250C and read after a minimum of 4 days incubation. (5 days for T. rubrum, T. mentagrophytes and T. tonsurans; 7 days for E. floccosum)
Reading results
The MIC was defined (except for Amphotericin) as the point at which there was 80% inhibition of growth as compared with the growth control when read visually in microtitre plates. For Amphotericin, the MIC was measured at 100% growth inhibition compared to growth control. MIC results recorded in μg/ml (CLSI M38-A2).
Quality control
Trichophyton mentagrophytes ATCC 4439 was procured from Sri Ramachandra Medical Centre & Research Institute, Chennai .RPMI 1640 medium, MOPS and antifungals were obtained from SIGMA ALDRICH, Egmore, Chennai
Data collected was entered into an excel sheet for further statistical analysis. The range of minimum inhibitory concentrations (MICs) were calculated for the microdilution method.
Results
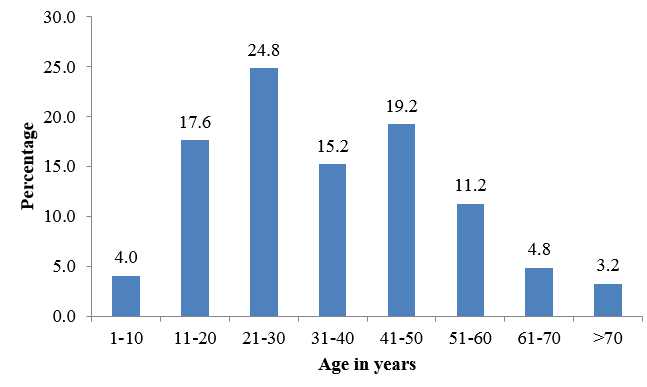
Out of the 125 samples collected, 117 were skin scrapings and 8 were nail clippings. There were no hair samples. Incidence of dermatophytosis was higher in females with male to female ratio of 0.58:1.This study showed that most cases of dermatophytosis was in the age group of 21-30 years (31 cases) followed by 41-50 years (24 cases). Least incidence was noted in age group above 70 year [Figure1].
Among males maximum number of patients were in age group 21-30 years and there were no cases reported in 70 years and above. Among females maximum number of patients were in 21-30 years and 11-20 years and least incidence in age group less than 10 years. Occupational profile of patients showed that largest group was house wives (42.4%), followed by students (25.6%). The next group was that of manual labourers(16%)46.4% patients have no similar history of illness in the past.45.6% patients have past history. 8.0% patients reported to have contact with cases.
Table 1
Sex distribution of clinical types of dermatophytosis
Table 2
Results of KOH study
|
KOH |
Number |
Percentage |
|
Positive |
76 |
60.8% |
|
Negative |
49 |
39.2% |
|
Total |
125 |
100% |
Table 3
Results of culture study
|
Culture |
Number |
Percentage |
|
Positive |
40 |
32% |
|
Negative |
85 |
68% |
|
Total |
125 |
100% |
Table 4
Relationship between direct smear positivity and culture positivity
|
KOH |
Culture |
Total |
|
|
Positive |
Negative |
|
|
|
Positive |
38 |
38 |
76 |
|
Negative |
2 |
47 |
49 |
|
Total |
40 |
85 |
125 |
Table 5
Culture positivity Vs clinical types
|
Clinical diagnosis |
Number |
Percentage |
|
Tinea corporis |
18 |
45% |
|
Tinea cruris |
18 |
45% |
|
Tinea pedis |
2 |
5% |
|
Tinea manuum |
2 |
5% |
|
Tinea unguium |
0 |
0% |
|
Tinea faciei |
0 |
0% |
|
Total |
40 |
100% |
Table 6
Minimum inhibitory concentration for various antifungal agents in µg/ml
Figure 4
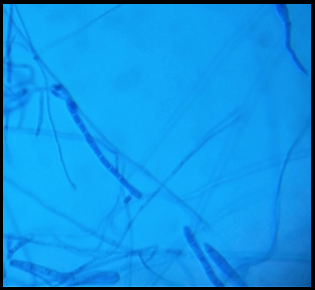
Koh preparation of skin scrapings; Septate hyaline hyphae of dermatophytes in wet mount (40X)

Figure 9
LCB mount of T. mentagrophytes; Spiral hyphae of dermatophytes as seen in Trichophyton. Mentagrophytes (40X)
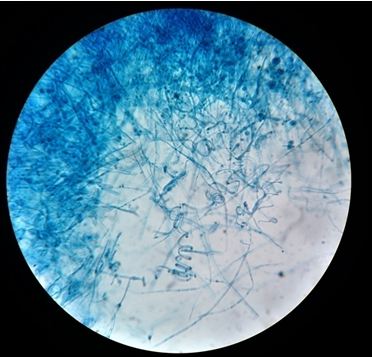
Figure 12
LCB mount of E. floccosum; E. floccosum showing clusters of club shaped macroconidia.(40X)
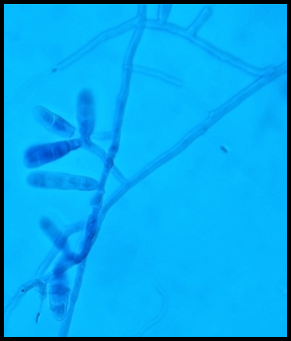
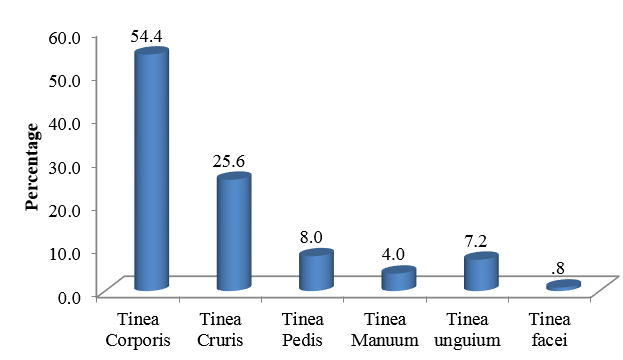
Most common clinical presentation was found to be Tinea corporis(54.4%), followed by Tinea cruris(25.6%). Tinea pedis(8%) was the next common clinical type followed by Tinea unguium(7.2%). The least common type in this study was Tinea faciei(0.8%)[Figure 2].
In this study, Tinea corporis was more common in males(56.52%). Tinea cruris(30.37%) and Tinea pedis(8.86%) were predominantly found in females. Tinea manuum(8.69%), Tinea unguium(8.69%) and Tinea faciei(2.17%) were predominantly found in males.[Table 1] Out of 125 samples, 76 samples were KOH positive(60.8%) and 49 samples were KOH negative(39.2%).[Figure 2] Out of 125 samples, 40 samples were culture positive (32%) and 85 samples were culture negative(68%).[Table 3]
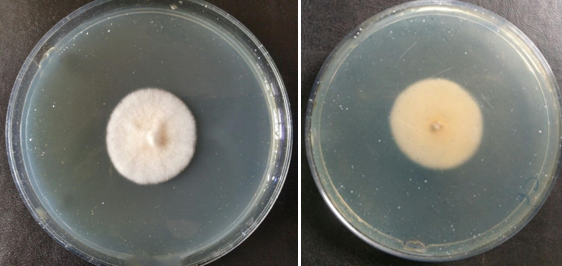
Out of 125 clinically suspected cases of dermatophytosis, 38 cases were positive by both microscopy and culture. 38 cases were positive by microscopy and negative by culture. Two cases were negative by microscopy but culture positive. Fourty seven cases were negative both by microscopy and culture.[Figure 4] Sensitivity of KOH positivity with respect to culture was 95% and specificity 55.3%. Positive predictive value and negative predictive value was 50% and 95.9% respectively. Maximum number of culture positivity was obtained from tinea corporis and tinea cruris. This was followed by tinea pedis and tinea manuum.[Figure 5] Out of 125 cases, isolation rate was 40(32%). Trichophyton mentagrophytes 20(50%) was the commonest species isolated followed by Trichophyton rubrum 14(35%), Trichophyton tonsurans 3(7.5%), Trichophyton verrucosum 2(5%) and Epidermophyton floccosum 1(2.5%).[Figure 3]
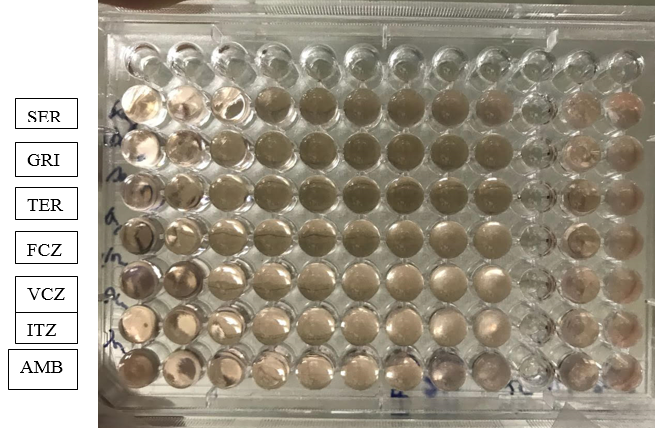
Antifungal susceptibility testing was carried out for T. mentagrophyes, T. rubrum, T. tonsurans, T. verrucosum and E. floccosum. Antifungals tested were Sertaconazole, Griseofulvin, Terbinafine, Fluconazole, Voriconazole, Itraconazole and Amphotericin B.[Figure 6] All the above mentioned MIC values of Sertaconazole, Griseofulvin, Terbinafine, Fluconazole, Voriconazole, Itraconazole and Amphotericin B were compared with the standard T. mentagrophytes ATCC 4439
Discussion
The present study highlights the clinical pattern and prevalence of different dermatophyte species implicated in tinea/ringworm infections in a tertiary care hospital in South Kerala.
Present study shows that out of 125 clinically suspected cases of dermatophytosis 40 (32%) were isolated by culture. Our results are comparable to studies conducted by Surekha 7 where the isolation rate was 30.8% and also studies conducted by Vikesh Kumar 8 where the isolation rate was 36.6%.
In the present study patients with age group 21-30 were commonly involved by dermatophytosis. Minimum incidence were found to be in extremes of age >70 years (3.2%) followed by age < 10 years (4%). In a study conducted by Madhavi in Hyderabad, India found out that out of 100 clinically suspected cases of dermatophytosis, the age group most commonly affected were 21-30 years. This is comparable with the studies conducted by Kamothi 9 Hanumanthappa 10 and Valarie. 11 Most of the other studies also showed high incidence of dermatophytosis in young patients. This may be due to greater physical activity, high incidence of trauma, increased sweating in these patients in addition to the tropical climatic conditions.
In the present study dermatophytic infection was more common in females (63.2%) and less common in males (36.8%). Male to female ratio was 0.58:1. This correlated with the results of Humera12 and Sweta et al13 where the male to female ratio was 0.85:1 and 0.74:1 respectively. In the present study Tinea corporis was diagnosed in 68 (54.4%) of 125 patients. Various studies conducted in South India by Srinivasan, 14 Lavanya15 and Hanumanthappa10 had reported 35.4%, 44.76% and 33.3% of Tinea corporis respectively. In the present study, KOH mount was positive in 76 (60.8%) and negative in 49 (39.2%) of the samples tested. Culture was positive in 40(32%) of the 125 samples tested, whereas 85 (68%) tested negative. This is in accordance with studies done by Valarie, Kamothi,9 Mahajan, 16 where they reported a direct microscopic positivity of 38.2% & culture positivity of 29.3%, 60% &48.5%, 79.6% &52% respectively. The relationship between KOH positivity and culture positivity was comparable with the results of Hanumanthappa.10 and Lavanya.15
The distribution of the dermatophytosis and their etiological agents varies with geographical location, the trends of people living in communities, and the socioeconomic conditions. In our study Trichophyton mentagrophytes was the commonest (50%), followed by Trichophyton rubrum(35%). These findings are consistent with findings of Venkatesh V N,17 where he noted that predominant dermatophyte was T. mentagrophyte 214 (13.46%) followed by T. rubrum 55 (3.46%). In the present study the prevalence pattern of dermatophytic fungi revealed T. mentagrophytes (9/18) 50% to be the most common isolate in cases of Tinea corporis. T. mentagrophytes (10/18) 55.5% was also the predominant species associated with Tinea cruris. In Tinea pedis, the fungi T. mentagrophytes and T. rubrum were equally isolated, one each. T. rubrum (100%) was the most common dermatophyte recovered from Tinea manuum.
Antifungal susceptibility testing was carried out for 39 isolates of Trichophyton spp and 1 isolate of Epidermophyton spp against 7 antifungal agents. Broth micro dilution method was used to determine the MIC for griseofulvin, fluconazole, voriconazole, itraconazole, terbinafine, amphotericin B and sertaconazole according to NCCLS (CLSI) M38A (2007) document for antifungal susceptibility testing for filamentous fungi with some modifications. In the present study both T. mentagrophytes and T. rubrum spp had a MIC range of <0.06-2μg/ml for terbinafine. Higher MICs for T. mentagrophytes was seen in 3 isolates(2μg/ml). This was comparable with studies of Sharma 8 where MIC range for sertaconazole was <0.06-4μg/ml. Out of 20 isolates of T. mentagrophytes and 14 isolates of T. rubrum, 6 isolates of the T. mentagrophytes and 4 isolates of the T.rubrum had low MIC value. And 6 isolates of T. mentagrophytes and 6 isolates of T. rubrum had high MIC of 4μg/ml and 2μg/ml respectively. MIC range of griseofulvin was 0.25-1μg/ml. Out of 20 isolates of T. mentagrophytes, 6 isolates had shown high MIC value of 1μg/ml and 3 out of 14 isolates of T. rubrum had MIC value of 1μg/ml. MIC value of both itraconazole and voriconazole had low range <0.06μg/ml and 0.06μg/ml except few isolates of T. rubrum which had 1μg/ml and 0.5μg/ml respectively. Among all the azoles, fluconazole had shown the highest MIC for all the isolates ranging from 2-8μg/ml. Similarly higher MIC range (2-8μg/ml) for amphotericin B was seen for most of the isolates.
Conclusion
Dermatophytes are a specialized group of fungi which affect keratinous tissue of humans and of other vertebrates, causing superficial infections. This study provides an assessment of the prevalence and etiological profile which could help in the estimation of the problem and prevention of spread of dermatophytosis with adequate control measures. Currently our study suggests itraconazole, voriconazole and griseofulvin are effective against dermatophytes. Irregular use of antifungal drugs has led to the emergence of resistant strains, which can cause poor treatment outcomes. Thus it is very important to test for antifungal sensitivity to check for resistance to antifungals.