Introduction
The rapid spread of coronavirus disease 2019 (COVID-19) worldwide led to more than seven hundred million confirmed cases and more than six hundred million deaths by March 2023 as reported in the WHO Coronavirus (COVID-19) Dashboard. 1 Bacterial coinfections, especially blood stream infections (BSIs) are common complications of many viral infections that lead to significant causes of morbidity and mortality in critically ill patients. 1, 2, 3, 4 Amongst hospitalized COVID-19 patients, admission in intensive care unit (ICU) were required for more than 20% of due to acute respiratory distress syndrome (ARDS) with another comorbidity. ICU patients are more susceptible to nosocomial infections during their stay and increase length of ICU stay, or the duration of mechanical ventilation. BSI are more common nosocomial infections associated with significant increased morbidity and mortality amongst in ICU patients. 3, 4, 5 To date increased frequency of bacterial co-infection in COVID-19 ICU patients and its severity of illness are not precisely elucidated. However, BSIs caused by MDRO and comorbidity in such cases are create grave situation in clinical outcome and consequently in treatment failure. Nonetheless, inappropriate use of antibiotics during COVID-19 is a potential source of the silent antimicrobial resistance [AMR] pandemic as reported in a few studies.6, 7 Sub-group analysis based on the key regions demonstrated that the prevalence of reported bacterial coinfection was higher in North America followed by Asia and Europe at 7.89%, 5.30% and 3.57%, respectively. Polymicrobial infections may play an important role in the development of SARS-CoV-2 infection in susceptible hosts. These coinfections may increase the risk of disease severity and pose challenges to the diagnosis, treatment, and prognosis of COVID-19. 8, 9, 10, 11 There are significant published Indian reports from early phase of the pandemic however recent information from India observing prevalence of bacterial coinfection and BSIs in COVID-19 is under reported subsequently, data included might not be up to date, which again, can compromise meta-analysis moreover from India. In this study, we aimed to determine the pervasiveness, comorbidity and associated risk factors, clinical significance and occurrence of etiological agents of BSIs in COVID-19 and its antimicrobial susceptibility for empirical antimicrobial therapy for positive clinical outcome.
Materials and Methods
This analysis of prospectively collected data was carried out in tertiary care hospital in Western India. Adult hospitalized patients with BSIs were admitted in intensive care unit (ICU) during COVID-19 wave from April 2020 to December 2021. COVID-19 was defined in the presence of a positive real-time polymerase chain reaction (RT-PCR) for SARS-CoV-2 on at least one respiratory specimen (nasopharyngeal swab, sputum and/or lower respiratory tract specimens.5 ICU-acquired BSI was defined in the presence of at least one positive blood culture for bacteria or fungi, drawn at >48 hours after ICU admission. For coagulase-negative staphylococci and other common skin contaminants, at least two consecutive blood cultures positive for the same pathogen were necessary to define BSI.5
Exclusion criteria
Age <18 years old.
ICU readmission.
Repeat blood culture isolates from a patient showing similar antibiotic susceptibility pattern as earlier isolate.
CLABSI (central line-associated bloodstream infection) is defined according to the NHSN (National Healthcare Safety Network) and CDC 2020 criteria, is a BSI in a patient that had a central line in place for 48 or more hours before the development of the BSI and is not related to an infection at another site. Primary BSI was determined if the patient did not have a clear source of infection whereas secondary BSI was defined by the identification of the same microorganism in blood culture and the suspected source of infection. BSI was classified as community-acquired if occurred within 48 hours of hospital admission and hospital-acquired if occurred after 48 h of admission. Polymicrobial BSI encompasses the identification of more than one species of microorganisms from a single positive blood culture.5, 8, 9, 10 In patients with multiple blood cultures positive for the same organism, novel BSI events were considered as independent if occurring at least 30 days after the last previous positive blood culture. Polymicrobial infections were considered as separate BSI events, one for each causative organism isolated from blood culture.5
Medical records for the source patient were reviewed for demographic information, history of prior hospitalization, and presence of major comorbid conditions (e.g. Diabetes mellitus, renal dysfunction, post-surgical status, malignancy, solid organ or stem cell transplantation, neutropenia, trauma or burn injury, history of hospitalization during the last 3 months before current admission, history of invasive procedures (gastroscopy, colonoscopy, bronchoscopy), medical history, history of antibiotic use, type and duration of antibiotics used, and finally therapies and laboratory findings related to COVID-19 infection. Correspond to survivors and non-survivors, respective physical characteristics that might concern mortality were moreover considered.
Microbiological investigations
Isolation and identification were done by standard conventional methods as well as automation system. Aerobic adult blood culture bottles (BD BACTEC Plus) received in Microbiology laboratory are incubated in BACTEC FX 40 blood culture instrument (Beckton-Dickenson, USA) at 35.5°C±1.5°C for 5 days. Blood culture samples which flash positive for aerobic bacterial growth are studied further to identify the isolate by sub-culturing on 5% blood agar and Mac-Conkey agar plates and further incubating aerobically at 37°C for 18-24 h. for bacterial isolation. Identification of isolate is done by routine microbiological & biochemical methods used in Microbiology laboratory and/or by using BD Phoenix M50 semi-automated system.
Antimicrobial susceptibility of these isolates is carried out as per CLSI guidelines 12 using NMIC ID/AST& PMIC AST panels as per isolate in BD Phoenix M50semi automated system. Antibiogram patterns of gram-positive and gram-negative bacterial isolates are studied separately to draw a conclusion regarding empirical choices of antimicrobials in these microorganisms. 12, 13, 14 MDRO was defined as acquired non-susceptibility to at least one agent in three or more antimicrobial categories. 15 Carbapenemase resistance and extended spectrum beta-lactamases [ESBL] (e.g. ceftriaxone, ceftazidime, ceftizoxime, and cefotaxime) isolates were classified recovered from bloodstream. 16
Consent and confidentiality
Separate consent of the patient was not essential as this is a study on the Blood culture samples collected as standard of care and study is based on secondary data. Blanket consent is taken as institutional policy. Confidentiality was maintained with no linkage of the data to the identity of an individual.
Results
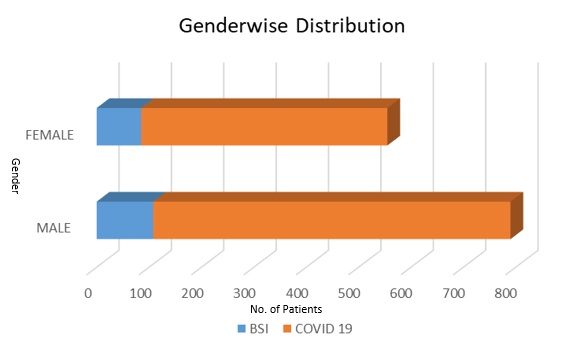
Of the 1152 patients admitted to the COVID-19 in ICU; 193 (16.7%) were developed BSIs (Figure 1) of which 7 (3.6%) were had candidemia and 186(96.3% were had bacteremia. In gender wise distribution majority were male (582/1152,59.2% BSIs 108/193, 48.1%) and female (470/1152, 40.7% and BSIs 85/193, 44%) (Figure 1)
Of 193 BSIs a substantial majority of patients had acute respiratory distress syndrome (ARDS) 81(41.9%) while chronic obstructive pulmonary disease (COPD) was in 46 (23.8%) patients.
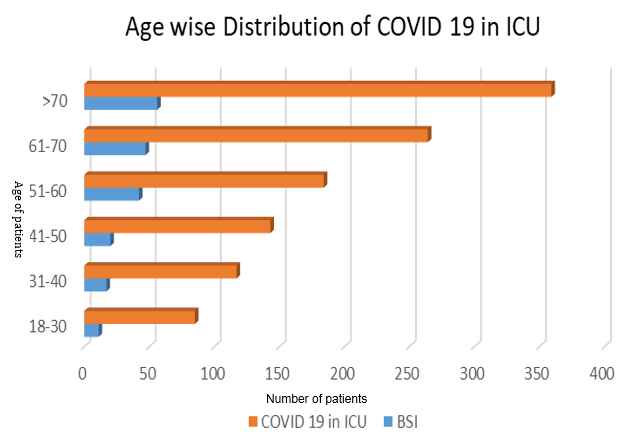
Age wise distribution amongst the COVID-19 patients admitted in ICU showed that patient from age 18 to 30 were 7.3% [85/1152, BSIs 11/193 (5.6%)], 31 to 40 were 10.1% [117/1152, BSIs17 (8.8%)], age 41 to 50 were 12.4% [143/1152], BSIs 21(10.8%)], age 51 to 60 were 15.9% [184/1152 BSIs 42 (21.7%)], age 61 to 70 were 22.9% [264/1152 BSIs 47 (24.3%)]and age more than 70 were 31.1% [359/1152 BSIs 56 (29%)] (Figure 2). Admission in ICU for COVID-19 were highest in elder patients followed by adults. BSI were also developed in elder patients predominantly. The elderly, especially those with underlying diseases, are more susceptible for COVID-19. Results demonstrated that the prevalence of the COVID-19 was higher in individuals ≥60 years of age than in younger individuals and subsequently due to underlying non- communicable chronic conditions, such as hypertension, diabetes, and cardiopulmonary diseases, were the most observed comorbidities. Invasive devices used in ICU patients such as foleys catheter, endotracheal intubation, arterial line, central venous line was also cause for developing BSIs in these patients.
Comorbidities and risk factors
Majority 638(55.3%) COVID-19 patients were having ARDS following COPD 572(49.6%) and Diabetes mellitus 522 (45.3%) and hypertension 141 (73%).Patients with invasive devices were more susceptible to for BSIs. Of the total 193 BSIs; 166(86%) COVID-19 patients were having Foleys Catheter, 134 (69.4%) were on Endotracheal intubation, 170 (88%) had Arterial line and 96 (49.7%) were having Central venous line in situ (Table 1). BSIs were developed in the patients who had different associate illnesses like Pneumonia 149 (77.2%), Bronchitis 93 (48.1%), Chronic Lung disease 81 (41.9%), Chronic Renal failure 107 (55.4%), Chronic liver disease 54 (27.9%), Ischemic heart diseases 77 (39.8%), Autoimmune 52 (26.9%), Malignancy 68 (35.2%). Of the total 1152 COVID-19 patients; 174 (15.1%) had multi-organ failure and all developed BSIs evidently.
Additional risk factors involved were poor nourishment, dehydration, and various clinical complications such as dementia, depression anxiety, disability and bedridden status of the patients.
Maximum COVID-19 patients clinically presented with more than one comorbidity and risk factors. The most common gram-negative clinical pathogens (GNCP) were Klebsiella pneumoniae 53(27.4%) followed by Acinetobacter baumannii 39(20.2%) and E. coli 27 (13.9%) and majority were MDRO. 12(6.2%) cases were having polymicrobial flora and 15 (7.7%) cases were identified having candidemia. Of the total 193, 149 (77%) pathogens were GNCP and 43(22%) pathogens were gram positive clinical pathogens (GPCP) followed by 15(7%) Candida spp. (Table 2).
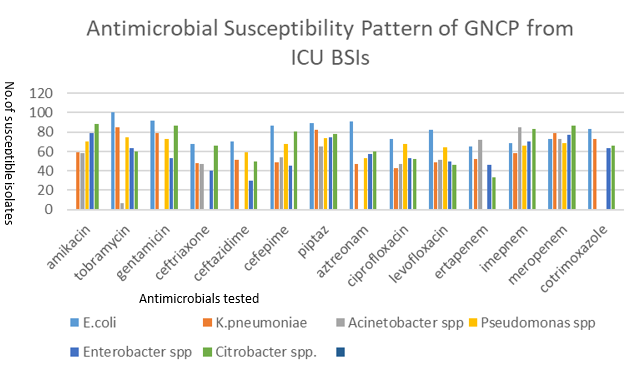
Among all pathogens, the highest resistance for Klebsiella pneumoniae was observed with ciprofloxacin (57%), aztreonam (53%) cefepime (54%), followed by ceftriaxone (52%) ceftazidime (49%) and meropenem (21%). In Acinetobacter baumanii resistance was observed against ciprofloxacin and ceftriaxone i. e. 53%. In Pseudomonas spp. aztreonam (47%), Ceftazidime (41%), levofloxacin (36%), imipenem (34% and meropenem (31%) showed higher resistance. In Enterobacter spp. Highest resistance was shown to ceftazidime (70%) and ceftriaxone (60%) and in Citrobacter spp. Ertapenem (66%), levofloxacin (64%), and ceftazidime (50%). Overall, 100% susceptibility was observed to tobramycin by E. coli and highest resistance was ceftazidime (70%) by Enterobacter spp. Amongst all GNCPs 149 (77%); 81(54.3%) isolates were MDRO and detected as MDRO by phenotypic screening test and were subjected for confirmation test.
The types of MDRO infections recognized in this study were CARBA and ESBL was in 81(54.3%) of which 60 strains were class A CARBA and ESBL, 45 strains were Class B MBL, 31strains were class C AmpC and 27 strains were class D OXA and 9 strains were MRSA of which 5 strains were MLSBi phenotype and 4 Enterococcus spp. were HLGR [Figure 3]. Many strains were carrying more than one MDR class of enzymes.
Overall, 100% susceptibility was observed to tobramycin by E. coli and highest resistance was ceftazidime (70%) by Enterobacter spp. Among all pathogens, the highest resistance for Klebsiella pneumoniae was observed with ciprofloxacin (57%), aztreonam (53%) cefepime (54%), followed by ceftriaxone (52%) ceftazidime (49%) and meropenem (21%) [Figure 4]
Figure 3
Number of MDRO strains isolated from BSIs from COVID-19 patients admitted in ICU. CARB: Carbapenemases, ESBL: Extended Spectrum β-lactamases, MBL: Metallo-β- lactamases, AmpC: AmpC beta-lactamases are clinically important cephalosporinases and mediate resistance to cephalothin, cefazolin, cefoxitin, most penicillins, and beta-lactamase inhibitor-beta-lactam combinations. OXA: Oxacillinases, OXA-type beta-lactamases confer resistance to ampicillin and cephalothin, MRSA: Methicillin resistance Staphylococcus aureus, MLSBi Phenotype: Resistance to macrolide, level gentamicin resistance (HLGR)
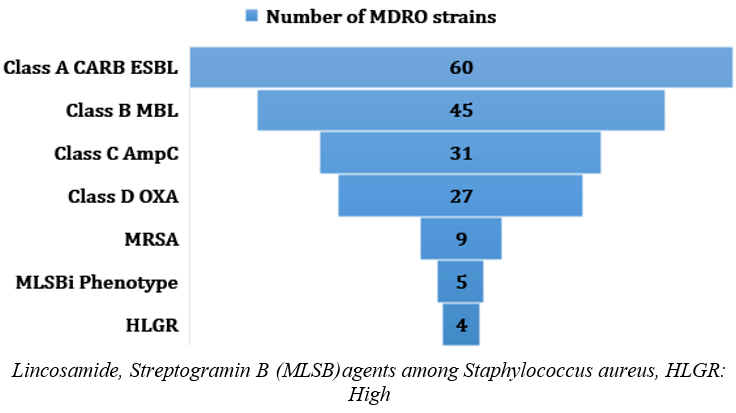
Table 1
Comorbidities and risk factors (%) of total BSI amongst COVID-19 ICU patients
Table 2
Profile of microorganisms and antimicrobial resistance isolated from BSIs from COVID-19 patents admitted in ICU.
Discussion
Published data concerning secondary bacterial BSI in ICU patients with COVID-19 are limited. In this study we found 16.7% overall prevalence of BSIs in admitted COVID-19 ICU (Figure 1) of which 3.6% were had candidemia and 96.3% were had bacteremia. In gender wise distribution 59.2% were male and BSIs in 48.1% while 40.7% female and BSIs in 44%(Figure 1). Naveenraj et al in 2021 reported 8.5% BSIs in ICU COVID-19 patients from western part of India. Vijay et al in 2021 reported 3.6% secondary infection in COVID-19 patients including hospital acquired as well as community acquired infections.17 Khurana et al in 2021 reported a 15% of severe illness in COVID-19 admitted in ICUs and secondary infections with resistant pathogens in COVID-19 patients and overall resistance ranged from 9% to 84% amongst all organisms.18, 19, 20 In the present study; of 193 BSIs a substantial majority of patients had acute respiratory distress syndrome (ARDS) 81(41.9%) while chronic obstructive pulmonary disease (COPD) was in 46 (23.8%) patients.
In the present study comorbidities are the main underlying aetiologies collectively ranging from 18% to 87.5% of cases (Table 1). Predominantly 55.3% ARDS, 49.6% for COPD, 45.3% for diabetes, 42.3% for hypertension.
Published investigation reports correlated to the impact of long-term conditions on COVID-19 has persistent on single comorbidities. Nevertheless, one-third of adults worldwide are projected to have two or more long-term conditions, increasing to more than two-thirds in those aged 65 or older. Agrawal et al from UK in 2022 reported around 58% of patients admitted to the hospital due to COVID-19 had multimorbidity.6 From a health-facilities viewpoint, clinically managing of patients with multimorbidity is frequently complicated. Multimorbidity be capable of strengthen the threat of destruction occurring through clinical intrusions. Elder age and underlying diseases have been noted as the main factors for vulnerability to COVID-19. An age ≥60 years is a major a risk factor. 3, 11, 21 In the present study, admission in ICU for COVID-19 were highest in elder patients followed by adults. Age wise distribution amongst the COVID- 9 patients admitted in ICU showed that patient from age 18 to 30 were 7.3% , age 31 to 40 were 10.1% , age 41 to 50 were 12.4%, age 51 to 60 were 15.9%, age 61 to 70 were 22.9% and age more than 70 were 31.1% [Figure 2]. Aging worsening of health-related issues to vital organs due to alter in functioning of pulmonary physiology, pathology during ling infections. Which affects responsiveness and tolerance in older patients. 22, 23, 24, 25 Overproduction of interleukin-6 (IL-6) in the brain in older age and increased expression of voltage-activated K+ channels, possibly increasing IL-6 production and neuroinflammation. Due to altered immune function through ion signalling can have profound impacts on the enhanced vulnerability to COVID-19 infection.
In the present study, further possible comorbidity were ischemic heart diseases [23.8%], chronic lung disease [15.6%], chronic renal failure 33.15%, Chronic liver disease [11.5%], Autoimmune [8.4%], malignancy [12%], multi-organ failure [15%] were reported. In this report we found significantly that infection related to invasive devices favoured in increasing complications in ICU COVID-19 patients (Table 1). In this study we observed BSIs related to invasive devices in COVID-19 ICU patients such as arterial line [88%], foley catheter [86%], endotracheal intubation [69.4%] and central venous line [49.7%] were recorded (Table 1). Other risk factors include poor nutrition, dementia, dehydration, and various clinical complications, especially in frail and bedridden patients. A lack of a timely diagnosis and therapeutic and preventive measures increases the risk of a severe infection. Restricted physical inactivity and produced social isolation-associated stress. These aspects may possibly added worsen the health of older people, causing to harmful health effects.
Diabetic patients have increased morbidity and mortality rates and have been linked to more hospitalization and intensive care unit (ICU) admissions. Patients with chronic obstructive pulmonary disease (COPD) or any respiratory illnesses are also at higher risk for severe illness from COVID-19 (CDC 2019). The risk of contracting COVID-19 in patients with COPD is found to be 4-fold higher than patients without COPD (CDC 2019). Generally, COVID-19 patients died due to the multiple organ failure. Clinical manifestations were reportedly more severe, and the disease course was more sustained in the aged, which necessitated rigorous medical supervision and interventions.9, 26
In the present study we found significantly predominant isolation of gram-negative clinical pathogens (GNCP) causing BSIs.
Nearly in 12(6.2%) cases of BSIs polymicrobial flora has been recorded. Related finding was described by Naveenraj P et al and Vijay S et al in their studies.17 However, a few studies have reported GPCP as predominant clinical pathogen causing BSIs in COVID-19.7, 27 In the present study, we found 7% candidemia and similar findings were reported by other studies Turkey, India and Brazil as well.28, 29, 30
We observed that in our patients scenarios, candidemia might have developed in patients before admission in ICU and or before hospitalization itself. Higher mortality has been observed in cases of Corticosteroid consumption, incidence of sepsis and old age patients. Candidemia with high mortality rates should always be kept in mind throughout the entire follow-up of ICU patients, even since the initial period. In the present study we observed the reasonably high percentage of Klebsiella pneumoniae 53(27.4%) and Acinetobacter baumannii 39(20.2%) followed by E. coli 27 (13.9%) and majority of them were MDRO (Table 2).
In the present study the high impact of COVID-19 on BSIs caused by MDRO GNCP in ICU have been observed. These results are concordant with the other earlier studies.31, 21, 26
Throughout the COVID-19 pandemic, several factors could have contributed towards the emergence and spread of multidrug resistance in hospitals as well as in community. These included excessive burden on hospital set ups specially ICUs and uncontrolled panic situations in hospital settings leading to patient-to-patient transmission. Moreover, the misuse and or overuse of antibiotics for suspected BSIs might have contributed to the emergence and spread of antimicrobial resistance. Almost all patients included in this study, received corticosteroids and immune suppressants along with broad spectrum antibiotics.
Limitation of the Study
Due to overload of detection and confirmation of COVID-19 cases; our study could not be extended to carry out genotypic characterization of ESBL, MBL and other CARBA enzymes, which would have given insight on dissemination of MDR strains.
Conclusion
Significant prevalence of MDR Klebsiella pneumoniae and Acinetobacter baumannii in BSIs of COVID-19 patients admitted in ICUs with multiple comorbid conditions suggest the crucial role of underlying acute clinical conditions, to which both the development of BSIs and mortality in these patients can be attributed. Appropriate initiation of antibiotic stewardship programme and strict compliance for prevention of infections and spread of antibiotic resistance is necessary. Damaging impact on disease management can be expected in seriously ill COVID-19 patients presenting with BSIs caused by MDROs.
Ethics Statement
The approvals by Institutional Research Committee, Independent Ethics Committee (Approval No.SIU/IEC/178), and Data Access Review Committee were obtained before the initiation of the study. The required norms of data privacy and confidentiality were followed, and Institutional Ethics Committee approved the study.