Introduction
Klebsiella. pneumoniae is a gram-negative rod belonging to the Enterobacteriaceae family that causes infections in humans depending on its site.1 This pathogen is known to cause pneumonia and urinary tract infections as well as other infections, including intra-abdominal infection, meningitis, pyogenic liver abscess, blood infection, and skin or soft tissue infections.1 Nevertheless, this bacterium is a commensal of the digestive system and can also be found in the environment. This organism often causes infections, mostly among hospitalized and immunocompromised individuals treated with ß-lactam antibiotics. 2 Cephalosporin- and carbapenem-resistant Klebsiella pneumoniae strains have been reported in South Africa to cause several mortalities in Johannesburg. 3 The prevalence of ESBL genes in human hospital disease samples isolated from Klebsiellla pneumoniae in Ghana is 64%.4 A study conducted in Cameroon reported that the percentage of ESBL-producing K. pneumoniae was 86.9%. 5 A study conducted in Kenya, ESBL-producing K. pneumoniae isolates were 92.2% and showed resistance to antibiotics, ceftriaxone (91.3%) amocillin/clavulanic acid (70.9%) cefepime (60.6%) nitrofurantoin (45.5%) azithromycin (22%) levofloxacin (12.6%) minocycline (11.8%) cefoxitin 9.4%.6
Urinary tract infections (UTIs) are the most common infections in primary care settings, affecting approximately 150 million people per year worldwide.7 The World Health Organization has ranked resistance to antimicrobials among the 10 most serious global public health crises facing healthcare at this time. 8 The WHO flags K. pneumoniae as an important antimicrobial (RAM)-resistant bacteria because of its strong tendency to develop resistance to current antibiotics, such as penicillins, cephalosporins and quinolones. 9, 10, 11 Carbapenems are considered to be the most effective antibiotics for treating multidrug resistance (MDR); however, K. pneumoniae isolates can be resistant to all three or more drug classes. 12 A high morbidity of 404.6 million urinary tract infections was reported in 2019; for this reason, monitoring and research are needed to reduce this healthcare burden. 13 In Africa, the prevalence of ESBLs in Enterobacteriaceae has been researched at the local level in various countries, but there is no summarizing research on how prevalent ESBLs are on the continent, what types of genes are involved and where research is missing. 14
Urinary tract infections pose a significant public health threat requiring significant economic implications. Due to the high empiric use of antibiotics for the treatment of UTI, antibacterial resistance of Enterobacteriaceae, specifically the main uropathogens Escherichia coli and K. pneumoniae, has significantly increased worldwide. 15 ESBL-producing Klebsiella strains are resistant to a number of antibiotics, such as aminoglycosides, fluoroquinolone, tetracyclines, chloramphenicol, trimethoprim + sulfamethoxazole, penicillins and cephalosporins.16 Carbapenems, such as imipenem, meropenem, and ertapenem, can only be used to treat infections caused by ESBL strains,17 although carbapenems should be avoided because they are used in cases of high antibiotic resistance infection.18 A study conducted in low- and middle-income countries reported a dramatic increase in the resistance of K. pneumoniae to amoxcillin/ampicillin (80%) and co-trimoxazole (67%).19
In sub-Saharan Africa, the resistance of K. pneumoniae to third-generation cephalosporins has increased from 8 to 77%.8 In Ethiopia, a study reported that K. pneumoniae isolated from urine samples presented 66.7% resistance to cotrimoxazole and 100% resistance to ampicillin.20 In Gabon, a prevalence of 16.2% in patients infected by the urinary tract has been reported for K. pneumoniae, and the bacterium has shown significant resistance to beta-lactams, quinolones and cotrimoxazole.21 In Nigeria, strong resistance of K. pneumoniae to ampicillin has been reported. 22 In Kenya, Kenyatta National Hospital (KNH) microbiology laboratory reported that isolated K. pneumoniae was resistant to all antibiotics used to treat childhood infections.23 Over the past two decades, the prevalence of K. pneumoniae has been 23%, and a high resistance of more than 80% to penicillins, cephalosporins, macrolides, tetracyclines, sulfonamides, lincosamides and chloramphenicol has been reported in Kenya.24 Affordable first-line agents such as ampicillin and gentamicin are unlikely to be clinically effective in a substantial proportion of infections. This has resulted in the increasing use of third-generation cephalosporins for the empirical treatment of serious infections.19
In Kenya, the multidrug resistance of K. pneumoniae has been reported to be greater than 80% for penicillins, chloramphenicol, cephalosporins, lincosamides, tetracyclines, macrolides and sulfonamides. Resistance to carbapenems was lowest at 23.2%, while resistance to amikacin, meropenem, aminoglycosides and quinolones was reported to be 21%, 7%, 49.2% and 41.3%, respectively. 24 This evidence shows that K. pneumoniae resistance is ever changing; thus, routine studies should be conducted to monitor it. There is also growing concern regarding the lack of new antibiotics, especially for multidrug-resistant gram-negative bacteria that produce ESBLs.25
This study therefore aims to help in understanding the trends of extended beta lactamase production in K. pneumoniae in UTI.
Materials and Methods
Study site
The study was carried out at Mama Lucy Kibaki Hospital between September 2023 and November 2023. Mama Lucy Hospital is a subcounty referral hospital providing comprehensive health care for both outpatients and inpatients within Nairobi City, Kenya. The hospital is located within Nairobi County between the Umoja-II and Komarock Estates of Nairobi, Kenya.
Study population
An estimated 400 participants aged between 18 years and 70 years were recruited into the study. The study targeted individuals presenting with UTI-like symptoms, including abdominal pain, frequent urination, back pain, cloudy urine, a burning sensation during urination, and a strong pungent smell. Those who consented to participate in the study were recruited while excluding those who did not consent and who were not able to produce urine or who were on antibiotic treatment.
Sample size determination
The sample size was determined using Fisher’s formula 26
N=Z 2 pq/d 2
where n= Sample size, Z= 1.96 at the 95% confidence interval, where the prevalence of UTI of 31.6% was considered; Q=1-P, D=degree of accuracy 0.05 at 95%, thus n=3.84∗0.316∗0.6847/ (0.05)2, n=400
The sample size was calculated on the basis of the 31.6% prevalence of K. pneumoniae mentioned in previous study conducted in Ethiopia. 27
Specimen collection
Sterile midstream urine samples were obtained from each consenting study participant under the instructions to urinate small amount into the toilet, then without stopping the flow of urine to med-stream urine in given sterile container up to 20mls. These samples were then sent to the CMR/KEMRI laboratory while kept 4oC. conditions for analysis within six hours of collection
Urinalysis
All 400 participants’ urine samples that were collected were subjected to macroscopy examination for color, smell, and consistency. Urine analysis was carried out using standard ComboStick 10 strips to assess the semiquantitative levels of leukocytes, pH, blood, nitrites, specific gravity and proteins in urine. 28
Urine culture
Using a sterile calibrated plastic loop, approximately 10 µl urine samples were inoculated on both MacConkey agar and Cystine Lactose Electrolyte Deficient (CLED) media and incubated at 37 °C. for 18–24 hrs. The bacterial or fungal isolates were identified on the basis of their culture characteristics. The presumptive bacteria were confirmed using Gram stain and specific biochemical tests. 29
Antimicrobial susceptibility test and ESBL detection
Approximately 0.5 McFarland’s standard pure bacterial isolates were inoculated to form a lawn on Mueller–Hinton agar. The antibiotic discs were distributed evenly on the agar surface and incubated at 37 °C. for 18–24 hrs. 30 The tested antimicrobials were ampicillin, cefotaxime, cefuroxime, ceftazidime, cefepime, imipenem, amoxicillin, clauvanalic acid, ceftriaxone, chloramphenicol, tetracycline, ciprofloxacin, sulfamethoxazole/trimethoprim and nitrofurantoin. The zones of inhibition were measured in millimeters by measuring the diameter of the zones using a ruler. The findings were compared to those obtained from controls followed guidelines for standard bacteria according to the Clinical and Laboratory Standards Institute. 31 The interpretation of the inhibition zone diameters was interpreted as susceptible, intermediate or resistant as per the Clinical and Laboratory Standards Institute guidelines. 31 The isolates that were resistant to cephalosporins and exhibited a synergy zone were further genetically analysed ESBL genes.
DNA extraction
The bacterial DNA extraction was performed by boiling method. The partial gene was amplified using specific primers (Table 1) in a 25 μL constituting of 5x buffer 5μl, template DNA 1 μl, DNTPs, MgCl2, taq polymerase, forward primer 0.5 μl, reverse primer 0.5 μl and nuclease-free water to 18μl under the following conditions; blaSHV at 940C for 30 seconds followed by 30 cycles at 94°C for 30 seconds, 50°C for 60 seconds, and 68°C for 1 min, with a final extension of 68°C for 5 min; blaTEM at 94°C for 30 seconds followed by 30 cycles of 94°C for 30 seconds, 50 °C for 60 seconds and 68°C for 1 min with a final extension at 68°C for 5 minutes, blaCTX-M at 94°C for 30 seconds followed by 30 cycles of 95°C for 30 seconds, 60°C for 60 seconds, 68°C for 1 min with a final extension at 68°C for 5 min and blaOXA at 94°C for 30 seconds followed by 30 cycles of 95°C for 30 seconds, 62°C for 60 seconds, 68° C for 1 min with a final extension at 68° C for 5 minutes. PCR amplification was confirmed via visualization with SYBR Safe DNA stain using gel electrophoresis. 32
Table 1
Klebsiella pneumoniae resistance genes and primers
Results
Demographic characteristics of study participants
The participants aged from 18 years to 70 years were recruited in the study. The age group30-35(33.5%) was the most predominant followed by age groups 24-29 (25.5%), 36-41(15.5%),18-2 (10.25%), 42-47 (6.5%), 48-53 (3%), 54-59 (2%), 60-65 (1.75%), respectively and the least was the age range of ≥66 at (1.5%). Females had a high number of participants 309(77.25%) compared to males 91(22.75%), and married participants were dominant compared to unmarried participants with 311 (77.75%) and 89 (22.25%), respectively, self-employed were at 169(42.25%) followed by employed with 155(38.75%) and the last were unemployed with 76(19%). The participants with secondary school level were the most dominant with 197(49.25%) followed by tertiary level with 191(47.75%), primary level with 10(2.5%) and the last was non-educated with 2(0.5%) Table 2.
Table 2
Demographic characteristics of the study participants
Dipstick positive and negative urine culture
Among 400 participants positive growth culture was observed in 206(51.5%) while negative growth was observed in 194(48.5%) Table 3
Prevalence of Klebsiella pneumoniae among UTI patients
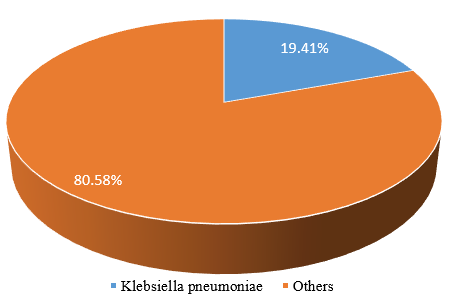
Figure 1 shows the isolated K. pneumoniae and others. K. pneumoniae isolates were 40(19.41%) while other isolates were 166(80.58%) from participants enrolled in this study.
Antimicrobial susceptibility patterns
From a total of 40 K. pneumoniae isolate that were tested, high levels of resistance was observed in Ampicillin (AMP) with 84.37%, followed by Ceftriaxone (CTR) and Cefotaxime (CTX) that had equal proportion of resistance 40.62%, Sulfamethoxazole-trimethoprim (COT) with 37.50%, Cefuroxime (CXM) with 34.37%, Tetracycline (TE) with 31.25%, Amoxicilin-clavulanic (AMC) with 28.12%, Ciprofloxacin (CIP) with 21.90%, Cefepime (CPM) with 12.50%, Nitrofurantoin (NIT) with 6.25% and the low resistance was observed in Chloramphenicol(C) with 3.12%. None of the isolates showed resistance to Imipenem (IPM). K. pneumoniae isolates were sensitive to Chloramphenicol 96.87%, Imipenem 93.75%, and Nitrofurantoin 90.62%, Ceftriaxone, Cefotaxime, Cefepime and Sulfamethoxazole-trimethoprim showed an equal sensitivity of 53.12%, Ciprofloxacin and Cefuroxime with 50%, and Tetracycline 46.87%. None of the isolate showed sensitivity to Amoxicilin-clavulanic. However multi-drug resistance (MDR) was observed to Cefotaxime, Ampicilin, Chloramphenicol, Imipenem and Cefuroxime (31.25%).Table 4
Table 4
Antimicrobial susceptibility patterns of K. pneumoniae
Among the 40 K. pneumoniae isolates, 30 (75%) were ESBL-positive. Figure 2
Resistance genes of Klebsiella pneumoniae
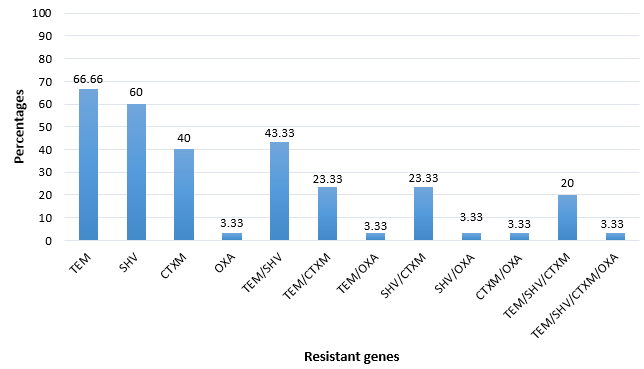
From the susceptibility profiles, those bacterial isolates that were β-lactamase resistant, their associated genes were determined. The frequency of these genes was; blaTEM (66.7%) followed blaSHV (60%), blaCTX-M (40%) with blaOXA (3.33%) as the least β-lactamase genes. The co-existence of multiple bla genes in a bacterial isolate was observed with blaTEM/SHV (43.33%) combined being the most common with blaTEM/SHV/CTXM/OXA as the least drug-resistant genes (3.33%). Figure 3
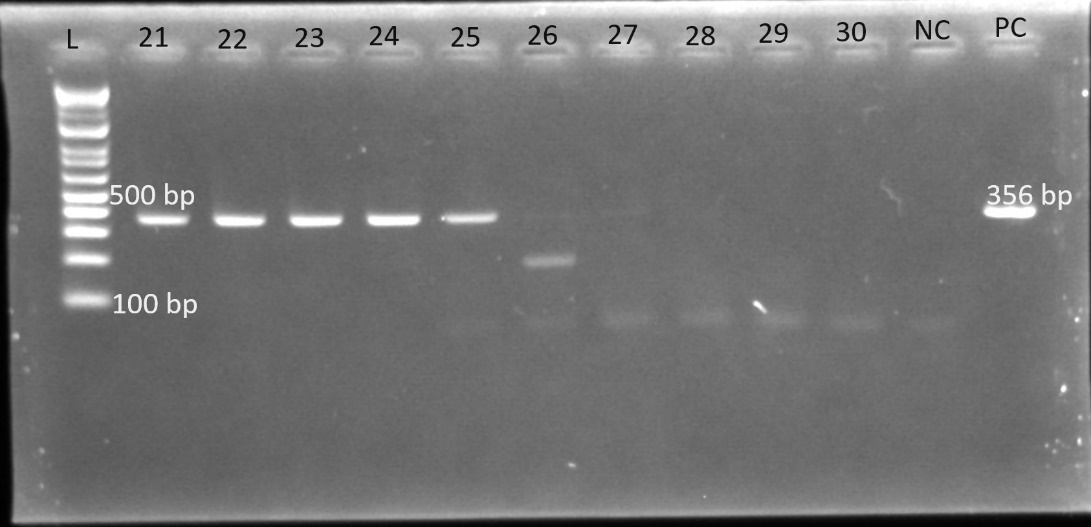
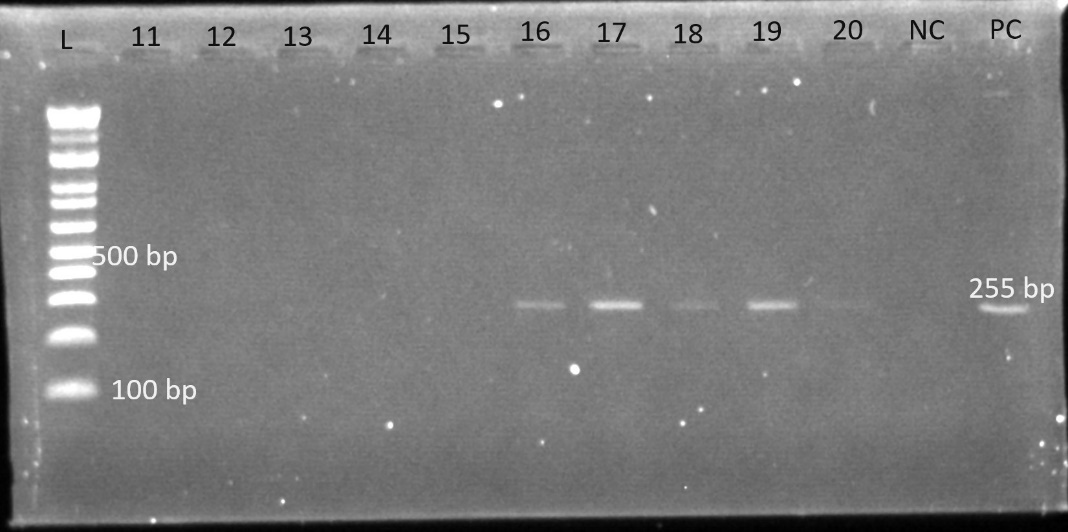
Gel electrophoresis was used to identify the bands. The gel plates below are representative electrophoresis gel images.
Discussion
Socio-demographic characteristics of the participants
In this study the majority of participants were females compared to males and the predominant age range was 30-35years (Table 1). In Kenya reported the same findings, where the number of female participants was greater than that of male participants.34 Another study conducted in Nigeria, reported the same findings, where the majority of participants were females (66%) rather than males (34%). 35 The findings of a study carried out in Uganda on UTIs revealed that there were more female participants (37.5%) than male participants 36, 21 reported that most participants were females (61.7%) and males (38.3%). Among women and men, urinary tract infection affects all age groups, with a higher incidence occurring among sexually active women. 37 Having external anatomical structure of reproductive organs that may favour the infection and shorter urethra closer to anus, expose females to UTI than males, because the bacteria ascendance from anus can easily enter through the urethra and invade the bladder. 37 Sexually intercourse is more common at 30 s, and at high frequency, this may be the cause. A contradictory study conducted by. 35 revealed that the majority of participants were in the age range of 22–26 years (36%). Other studies contradicted the findings of,36, 38, 39 who reported that the most prevalent age ranges were 16–30 years (37.9%), 20–29 years (32.6%), and >45 years (25%), respectively. In Ethiopia, a study on the prevalence of bacterial urinary tract infections reported a high occurrence of the disease among those in the 20–69, years groups,27 which is in agreement with the findings of the current study, where people in the mentioned age interval were more prone to UTIs. Compared with single individuals, married individuals are reported to be more affected by UTIs,40 which may be due to the frequency of transmission of infection among couples, which contributes to a significant number of married women having UTIs than men do.27
Prevalence of Klebsiella pneumoniae among UTI patients
The prevalence of Klebsiella pneumoniae reported in the present study was 19.41% (Figure 1 ), which is lower than the prevalence reported in a previous study carried out in Kenya which reported prevalence of 33.3% Klebsiella pneumonia.34 In Kenya study conducted reported that 29% was contributed to Klebsiella pneumoniae in UTI.41 Another study carried out in Bangladesh reported a prevalence of 21.6% Klebsiella pneumonia.42 A study conducted in Kenya showed a prevalence of Klebsiella pneumoniae which stood at 8.9%.43 In Uganda, a prevalence of 11.6% was reported.36 Among the Klebsiella species contributing to UTIs in Northwest Ethiopia, Klebsiella pneumoniae was the most common isolate, with more than twice the number of other Klebsiella species isolated. 38 its prevalence was 65.8%, which is higher than what the current study has determined. Similarly, a high prevalence of Klebsiella pneumoniae was observed in the U.S. and represented more than half of all Klebsiella spp. isolated from urine samples. 44 This may be due to poor hygiene and wiping back to front rather than front to back. 45
Among community uropathogen isolates in Gabon, Klebsiella pneumoniae accounts for approximately 16.2% of the total prevalence of UTIs, making it the second most common isolated microorganism among patients.21 Another study conducted in Saudi Arabia reported that Klebsiella pneumoniae was the most commonly observed isolate among its genera, accounting for 97.4% of the Klebsiella spp. identified in urine.46 With its ability to invade soft tissues, Klebsiella pneumoniae uses its type 1 fimbriae in the attachment and invasion of bladder epithelial cells, which may also favour the formation of biofilms supporting its long survival in the bladder, leading to its high prevalence in chronic UTIs.47 Among the Klebsiella pneumoniae isolates, the high prevalence of ESBL-producing Klebsiella pneumoniae was 75% (Figure.2).
A study conducted in Cameroon reported a prevalence of Klebsiella pneumoniae of 82.4%.5 In Turkey, a similar study on urinary tract infection reported a high prevalence of ESBL-producing Klebsiella pneumoniae, which was 78.8%.48 Another study carried out in Nigeria reported a prevalence of 31% for ESBL-producing Klebsiella pneumonia,35 which is very low compared with the prevalence reported in the current study. In the Tertiary Care Hospital of Central Nepal, the total reported prevalence of K. pneumoniae was 223 (11.70%), 19% of which were ESBL producers.49 In Iran, the percentage of ESBL-harboring K. pneumoniae was 13.5%,50 while it was reported to be 31% in a study carried out in Nigeria. 35 In Kenya, a study carried out in two referral hospitals reported a high prevalence of ESBL-producing K. pneumoniae, which was 92.8%.6 K. pneumoniae was reported as one of the top ESBL producers among Enterobacteriaceae, which supports its high occurrence in the chronic phase of urinary tract infection.51
Antimicrobial susceptibility patterns and ESBL producing K. pneumoniae
In the 20th century, the discovery of antibiotics has played a significant role in the treatment of microbial diseases 52, and antimicrobial resistance resulting from the high selection of antibiotics for treatment and their misuse and overuse has presented a global public health challenge for health systems in recent decades. 53 The present study revealed high resistance (84.37%) of this uropathogen to ampicillin and MDR phenotypes to cefotaxime, ampicillin, chloramphenicol, and cefuroxime, whereas the highest sensitivity was observed for chloramphenicol, imipenem, and nitrofurantoin (Table 2).
Similar findings were reported in India where K. pneumoniae showed the highest resistance to ampicillin (75.6%), nitrofurantoin (73.1%), and cefuroxime.54 Additionally, all ESBL-producing K. pneumoniae strains were reported to be MDR, while they showed high resistance to nitrofurantoin and contrimonazole.54 In Morocco, differences in resistance for both non-ESL-producing K. pneumoniae and ESBL-producing K. pneumoniae were detected for sulfamethoxazole-trimethoprim (61%, 89%), ciprofloxacin (32%, 84%), gentamicin (21%, 89%), and amikacin (11%, 50%), with the highest resistance reported for ESBL-producing K. pneumoniae rather than non-ESL-producing K. pneumonia. 55 The Klebsiella pneumoniae ESBL producer was sensitive to imipenem (96%), as reported by 5 in Cameroon. In the Kurdistan Region of Iraq, K. pneumoniae has been reported to be highly resistant to ampicillin (96.9%), ceftriaxone (65.8%), and cefepime (60.8%), whereas the highest sensitivity was observed for ertapenem (93.8%), and 82.3% was attributed to mipenem. 56
In Egypt, a study conducted in a tertiary care hospital showed that 90% of ESBL-producing Klebsiella pneumoniae strains were resistant to Sulmethoxazole/trimethoprim, 70% were resistant to amoxicillin/clavulanate, 63.3% were resistant to cefotaxime, 40% were resistant to cefepime, 46.7% were resistant to ceftriaxone, and the least resistance is observed for imipenem.57 Another study conducted in Italy reported the broad resistance of K. pneumoniae to penicillins and cephalosporins, whereas high resistance of ESBL-producing K. pneumoniae to carbapenem antibiotics was reported.58 Similar findings revealed 100% resistance to ampicillin, whereas 70–80% resistance was reported for first-, second-, and third-generation cephalosporins. However, high sensitivity to imipenem has been reported. 59
The present study reported the high resistance to ampicillin, in agreement with study conducted in Bangladesh, the majority of K. pneumoniae strains (82%) are MDR and were resistant to antibiotics, including β-lactam antibiotics, aminoglycosides, carbapenems, and ciprofloxacin 60 The uniform sensitivity of K. pneumoniae to imipenem was reported in a study carried out in India, but the study also revealed high susceptibility to β-lactamase combined drugs (67–81%) and aminoglycosides (62–76%); however, the lowest sensitivity was observed for third-generation cephalosporins (14–24%) and non-β-lactam antibiotics, including nitrofurantoin (57%), fluoroquinolones (29–57%), piperacillin (19–23%), and aztreonam. 61
Another study conducted in Iran revealed that all ESβL-producing K. pneumoniae strains were sensitive to imipenem and meropenem, and the isolates were resistant to aztreonam. Significantly high resistance to amoxicillin (100%), cefotaxime (50%), and gentamicin (42.3%) was observed, whereas the least resistance to imipenem (15.9%) and meropenem (11.8%) was observed. 62 Approximately 99% of ESβL-producing K. pneumoniae were resistant to sulfonamides; 81% were resistant to quinolones, whereas 79% were resistant to aminoglycosides. 51 In the last two decades, a significant decrease in the susceptibility of K. pneumoniae to third-generation cephalosporins and ciprofloxacin was reported, with sensitivities of 83.6% and 81.6% attributed to cefotaxime and ciprofloxacin, respectively, in 2012. 63
Another study conducted in Kenya reported that, compared with other isolates, Klebsiella pneumoniae was more resistant to nitrofurantoin. 64 A study conducted in Benin reported that ESBL-producing Klebsiella pneumoniae was highly resistant to amoxicillin (79.07%), cefotaxime (53.48%), and trimethoprim/sulfamethoxazole combinations (86.05%). 65 In Bagdad, Iraq, high resistance of K. pneumoniae was detected to antibiotics such as ampicillin (100%), cefixime (73.8%), cefuroxime (71.05%), and ceftazidime (65.79%), and intermediate resistance was reported to antibiotics such as nitrofurantoin, sulfamethoxazole-trimethoprim, and ceftriaxone, while the lowest resistance was observed to antibiotics, including imipenem, meropenem, and ciprofloxacin. 66 In Ethiopia, K. pneumoniae has been isolated from other Enterobacteriacieae and has shown high resistance to cotrimoxazole (91.7%) and chloramphenicol (66.7%), whereas other types of resistance have been reported for ciprofloxacin (45.8%), norfloxacin (45.8%), and 25% resistance to gentamicin. In terms of MDR, this uropathogen (K. pneumoniae) has 58% MDR among other Enterobacteriacieae. 67
Other findings reported in Gaza in Palestine, the high resistance of K. pneumoniae was observed to antibiotics such as Cefotaxime, ampicillin, and Sulfamethoxazole-trimethoprim.68 similarly, another study reported the high resistance of K. pneumoniae to ampicillin, Sulfamethoxazole-trimethoprim, Cefotaxime, piperacillin, levofloxacin, gentamicin, ceftazidime, ceepime, and aztreonam, although, the moderate resistance was observed to ciprofloxacin and ceftriaxone, while the least resistance was detected for imipenem. 69 It was also observed in another study that K. pneumoniae isolated from was 90% MDR and all MDR K. pneumoniae were ESBL- producers. 70
Extended-spectrum β-lactamase resistance genes in Klebsiella pneumoniae
One of challenges in the use of β-lactam antibiotics for treatment of bacterial infections is the production of Extended-spectrum β-lactamases enzyme by majority pathogens of which K. pneumoniae was ranked among the top. However, the production of this enzyme (ESBL) was found to be a result of gene expression from bacterial genome. 71 In this study, we have observed a high occurrence of blaTEM and blaSHV, the moderate occurrence of blaCTX-M, and the least occurrence was observed to blaOXA. The combination of bla genes was also studied (Figure 1). 72 has reported the similar findings where blaTEM (49.4%) was reported as the most common genotype detected, in contrast, blaSHV was observed as the least detected genotype in K. pneumoniae.
The same bla genes reported in a study carried out in Malaysia contradicted the findings of the present study: blaSHV was detected in 46 K. pneumoniae isolates for blaSHV, 19 isolates for blaCTX-M, 5 isolates for blaOXA, and 4 isolates for blaTEM. 73 In Southwest Nigeria, different percentages of K. pneumoniae bla genes have been reported, and all the genes detected in the present study were also detected in Nigeria at different percentages. The bla genes detected included blaTEM (47.7%), blaCTX-M (43.8%), blaSHV (39.8), and blaOXA, which were the least frequently detected and represented 27.3% of all genes. 74 Another study revealed that blaCTX-M occurred in 30% of isolates, whereas other bla genes were not detected. 75
Similar bla genes were reported in a study conducted in Egypt 10% of Klebsiella pneumoniae isolates was for blaSHV and 53.3% for blaCTX-M. 57 Klebsiella pneumoniae isolated from hospital in Benin reported the occurrence of blaSHV and blaCTX-M. 65 Differently, a study carried out in China, showed the high occurrence of blaSHV which was the most prevalent, and the second prevalent was blaTEM, while the least prevalent reported bla gene was blaCTX-M with zero to blaOXA. 76 Again, another study in China, reported the high prevalence of blaTEM in ESBL-KP which stood at 69.3%, blaCTX-M was 45.5% and the least observed was blaSHV which stood at 4.5%, no blaOXA was also reported for this study.77 In Iran, ESBL-producing K. pneumoniae showed the presence of 59.3% for blaTEM while the presence of the combination of blaCTX-M/ blaTEM was observed at 33.3%.78 In Nepal, the overall prevalence of blaCTX-M was 89.62% in which K. pneumoniae had 78.94%. 79 In hypervirulent ESBL-producing K. pneumoniae, blaSHV (63.8), while 59% and 58.1% were attributed to blaTEM and blaCTX-M respectively. 80
ESBL bla genes were also found and described in 32 ESBL-producing K. pneumoniae strains, where blaCTX-M genes were detected in 20 isolates. blaSHV genes were detected in 2 isolates, whereas combinations of blaCTX-M genes and blaSHV genes were detected in 9 isolates, with an unknown gene that was observed in 1 isolate. 81 The overall prevalence rates reported for blaTEM, blaCTX-M, and blaSHV were 86%, 78%, and 28%, respectively, of which ESBL-producing K. pneumoniae carried 34% for blaTEM, 31% for blaCTX-M, and 26.1% for blaSHV genes. 82 In Kenya, the predominant ESBL genes reported for K. pneumoniae were blaTEM, which stood at 89%; blaSHV, which stood at 82.7%; blaOXA, which stood at 76.4%; and blaCTX-M, which stood at 72.5%. In addition, the presence of the blaSHV, blaOXA, and blaTEM genes were associated with MDR. 6
Another contradictory study was carried out in Kenya, where a high occurrence of blaCTX-M genes was observed, followed by blaTEM, and the least detected bla gene was blaOXA.41 A study in which samples from both Kenya and Uganda were collected detected K. pneumoniae resistance bla genes, which included blaCTX-M genes, blaTEM genes, and blaOXA, the K. pneumoniae strains with these genes were 100% resistant to 3 more antibiotics, revealing the trend of MDR for these bacteria and predicting future pandemics resulting from this threat if nothing is done.83 In Nigeria, the two K. pneumoniae isolates harboured both blaSHV and blaCTX-M of the blaCTX-M-1 group, the third K. pneumoniae harboured only blaCTX-M of the blaCTX-M-1 group, and the study revealed the critical threat of the increase in carbapenem-resistant K. pneumoniae resulting from coharbouring both blaCTX-M of the blaCTX-M-1 group and blaSHV genes. 84
In Eastern province, South Africa, ESBL-producing K. pneumoniae (n=139) harboured the high prevalence of blaSHV (n=22) for the single ESBL genes, but also showed blaTEM (n=5), while blaCTX-M was observed at low frequency in this category, for two or more ESBL genes, K. pneumoniae had 78/139 for blaTEM + blaSHV + blaCTX-M, 12/139 for blaTEM + blaSHV, 6/139 for blaCTX-M + blaSHV, and 4/139 for blaCTX-M + blaTEM, no blaOXA gene was observed in the study.85 A study carried out in three countries (Egypt, Saudi Arabia, and Sudan) revealed that blaCTX-M was detected in all K. pneumoniae isolates while blaTEM was reported at 66.7%, the presence of these genes highlighted the high MDR in mentioned MDR to the last line antibiotics.86 Another similar study reported the high prevalence of blaTEM genes which stood at 57.1%, blaCTX-M had 28.6%, some genes were occurred in combination within K. pneumoniae genotype, and include blaSHV and blaCTX-M which stood at 14.3%, blaTEM, blaSHV, and blaCTX-M stood at 14.3%.87
Limitations
Due to the lack of resources the advanced molecular method which show whether the genes found were plasmid or chromosomal associated was not done.
Conclusion
The prevalence of K. pneumoniae was 19.40%, 75% of which were ESBL producers. This uropathogen showed high resistance to ampicillin and high susceptibility to chloramphenicol, imipenem, and nitrofurantoin. Multidrug resistance (MDR) to antibiotics such as cefotaxime, ampicillin, chloramphenicol, and cefuroxime has been detected. The observed ESβL resistance genes in K. pneumoniae included blaTEM, blaSHV, blaCTX-M, and blaOXA, with blaTEM being the most prevalent. The successful completion of this study highlighted the emerging resistance of K. pneumoniae among urinary tract-infected patients. Thus, this study recommends the need for that healthcare facilities for routine laboratory testing for ESBL phenotypic and molecular UTI diagnoses to guide clinical treatment of UTI patients and AMR need for regular surveillance for the emergence and spread of ESBL among UTI-causing KP.