Introduction
Otomycosis is prevalent in humid environments and often involves fungi such as Aspergillus and Candida. While Aspergillus species are common, Aspergillus nidulans is seldom reported. Effective diagnosis relies on KOH preparation and culture, which are essential for managing this condition. This report details a case of otomycosis caused by Aspergillus nidulans, with an emphasis on the diagnostic and treatment processes. 1
Case Presentation
Chief complaint
A 45-year-old female presented with persistent itching, discomfort, and discharge from the right ear over the past three weeks. The patient's symptoms began in mid-June, during the hot and humid summer season.
Occupational and Socioeconomic Background: The patient works as a farmer, frequently exposed to dust, soil, and moisture, all of which are potential sources of fungal spores. Additionally, the patient's socioeconomic background includes limited access to healthcare facilities, which may have delayed diagnosis and treatment, further predisposing her to the infection.
Family history
The patient’s sibling had a history of recurrent fungal infections, although these were not specified as Aspergillus or other fungal infections.
Past medical history
The patient had no significant medical history of chronic diseases or previous ear infections.
Physical examination
Examination of the ear revealed white, fluffy fungal debris in the right external auditory canal, accompanied by mild erythema of the canal walls. The tympanic membrane was intact.
Sample collection
A sterile swab was used to collect fungal debris, although a sterile swab was used to collect the fungal debris from the right external auditory canal, swab collection is generally considered the least satisfactory method in microbiology. Ideally, visible fungal debris should have been collected directly in a sterile container for more accurate results. However, in this case, swabbing was necessary as the entire debris could not be obtained in a single collection, and the swab allowed for adequate sample transfer for culture.
Laboratory diagnosis
KOH preparation
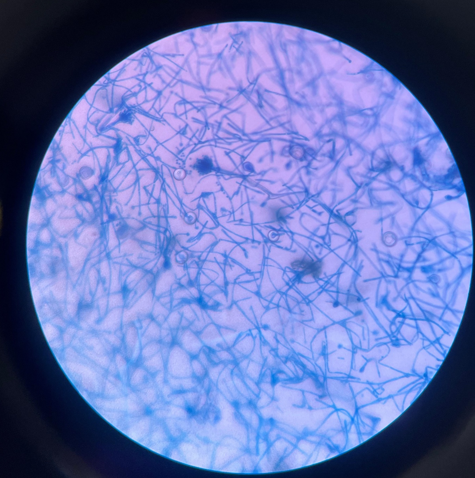
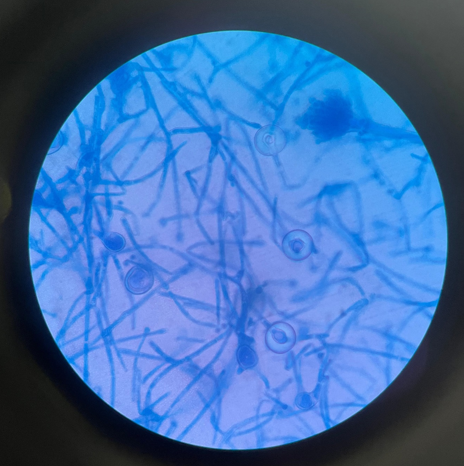
A 10% potassium hydroxide (KOH) preparation showed septate hyphae with acute angle branching, indicative of Aspergillus species. (Figure 1)
Fungal culture
The swab allowed for adequate sample collection, which was subsequently cultured on Sabouraud Dextrose Agar (SDA) for further fungal identification. The plates were incubated at 25°C and 37°C for 7 days, with daily monitoring for colony growth and fungal identification.
Initially yielded white colonies, which later turned greenish and powdery.
Macroscopic examination
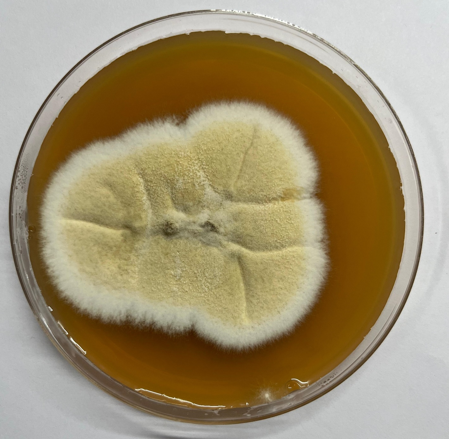
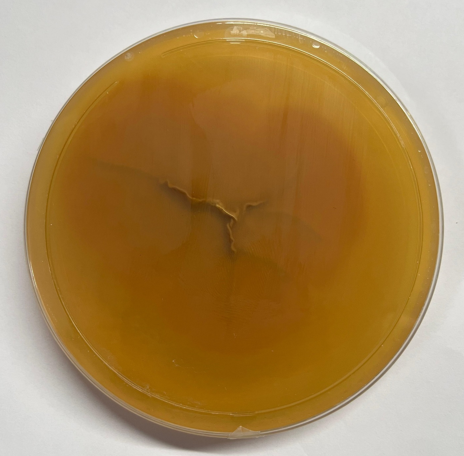
The colonies on SDA displayed an initial white appearance, evolving to a greenish color with a powdery texture. The reverse side of the plate showed a yellowish-green coloration, characteristic of Aspergillus nidulans pigmentation. (Figure 2, Figure 3)
Microscopic Examination (LPCB Mount)
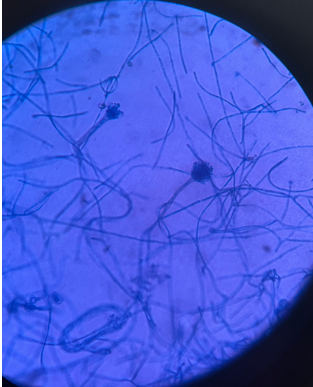
Lactophenol Cotton Blue (LPCB) mount revealed septate hyphae with unbranched conidiophores that terminated in vesicles. These vesicles gave rise to phialides arranged in a biseriate pattern, producing chains of round, smooth-walled conidia. Additionally, the presence of Hulle cells, a distinguishing feature of Aspergillus nidulans, was observed, confirming the species identification. (Figure 4, Figure 5, Figure 6)
Identification
The identification of Aspergillus nidulans was based on KOH preparation, fungal culture morphology, and LPCB mount. Hulle cells were crucial for confirming the diagnosis.2
Antifungal susceptibility testing
Antifungal susceptibility testing was not performed, relying instead on established susceptibility profiles for Aspergillus nidulans. Standard treatment protocols were followed.3
Figure 1
Microscopic image of aspergillus nidulans under light microscopy using 10% potassium hydroxide (KOH) solution

Treatment
The patient was treated with topical clotrimazole ear drops twice daily for two weeks and oral itraconazole (100 mg daily) for 10 days. While this empirical treatment resolved the symptoms, conducting a sensitivity test would help confirm the most effective antifungal agent for Aspergillus nidulans in this case. Future management of similar cases could benefit from incorporating sensitivity testing to tailor the treatment. Additionally, trying new antibiotics or antifungal agents could be explored to discover more effective treatments, particularly in cases where resistance to standard therapies is a concern.
Discussion
Otomycosis caused by Aspergillus nidulans is rare but should be included in differential diagnoses for chronic ear infections. Accurate diagnosis requires comprehensive mycological examination, including KOH preparation, LPCB mount, and fungal culture. The identification of Hulle cells was key in confirming Aspergillus nidulans.
While the case was successfully treated using empirical antifungal therapy based on known susceptibility patterns, conducting antifungal susceptibility testing could further refine treatment choices. Sensitivity testing would provide personalized insights into which antifungal agents are most effective against the specific isolate. This becomes particularly relevant as emerging resistance patterns in Aspergillus species make treatment more challenging. Additionally, exploring new antifungal agents or antibiotics for Aspergillus nidulans could yield more effective options for treatment, especially for resistant or recurrent infections. 4
The incidence of Aspergillus nidulans as a causative agent of otomycosis is relatively low compared to other species within the Aspergillus genus. In a study conducted in Iran, it was found that among various fungal isolates from patients with otomycosis, Aspergillus species accounted for approximately 85% of cases, with A. niger and A. flavus being the predominant species. Specifically, A. nidulans was reported infrequently, highlighting its rarity in clinical presentations of ear infections. 5
These findings emphasize the importance of comprehensive mycological assessments in cases of chronic ear infections to accurately identify less common fungal pathogens like Aspergillus nidulans.6
Limitations
Lack of Molecular Identification: Molecular techniques, such as PCR-based methods, were not used to confirm the fungal species. These methods could enhance the accuracy of identification and differentiate Aspergillus nidulans from other similar species.
Absence of Antifungal Susceptibility Testing: Antifungal susceptibility testing was not performed. Although treatment was based on known susceptibility patterns, such testing could provide more specific guidance and ensure the effectiveness of the chosen antifungal agents.
Lack of Exploration of New Antibiotics: The case report did not explore the use of new or alternative antibiotics for treating Aspergillus nidulans. Conducting trials with new antifungal agents could help identify more effective options, especially for resistant or recurrent infections
Single Case Study: This report is based on a single case, limiting the generalizability of the findings. Larger studies are needed to better understand the prevalence and clinical significance of Aspergillus nidulans in otomycosis.
Limited Follow-Up Data: The follow-up period focused solely on symptom resolution. Long-term follow-up data on recurrence rates or potential complications were not collected, which could be important for assessing the long-term outcomes of treatment.