- Visibility 70 Views
- Downloads 5 Downloads
- DOI 10.18231/j.ijmmtd.2021.020
-
CrossMark
- Citation
A comparative study of Widal test with blood culture in the diagnosis of typhoid fever in febrile patients
- Author Details:
-
Pooja Choubey *
Introduction
Typhoid fever is an acute illness associated with fever caused by the Salmonella enterica serotype Typhibacteria. It can also be caused by Salmonellaparatyphi.
After the ingestion of contaminated food or water, the Salmonella bacteria invade the small intestine and enter the bloodstream temporarily. The bacteria are carried by white blood cells in the liver, spleen, and bone marrow.
Typhoid fever is treated with antibiotics which kill the Salmonella bacteria. With antibiotics and supportive care, mortality has been reduced to 1%-2%. Appropriate antibiotic therapy, there is usually improvement within one to two days and recovery within seven to 10 days. For those travelling to high-risk areas, vaccines are now available.[1]
Symptoms
The incubation period is usually 1-2 weeks, and the duration of the illness is about 3-4 weeks. Symptoms include:
Poor appetite
Headaches
Generalized aches and pains
Fever as high as 104 degrees Farenheit
Lethargy
Diarrhea
Materials and Methods
The culture bottles were incubated at 37°C. Incubation was continued for 7 days unless the visible growth was obtained. After each day of incubation blind subculture were done on Blood agar (BA), Chocolate agar (CA) and Mac Conkey agar (MA) up to seven days of incubation. The day of collection of sample was defined as the first day in this study.[2] The identification of bacteria from isolated colonies was done by standard microbiological procedures as described in Bergey’s Manual, which involve colony morphology, Gram stain and biochemical reaction. Various biochemical media were inoculated and the results were observed on following day. [3]
Study area and period
The study of conducted in red cross hospital at Madhya Pradesh, Bhopal from mid of May 2018 to August 2018
Study design and patient population
This study is conducted in department of microbiology on Red Cross hospital at Bhopal. Total population of patients is 62 were observed.
Blood culture
For the culture, a small sample of your blood, stool, urine or bone marrow is placed on a special medium that encourages the growth of bacteria. The culture is checked under a microscope for the presence of typhoid bacteria. A bone marrow culture often is the most sensitive test for A prospective study on febrile patients was conducted in which patients were screened for typhoid fever and suspected patients were enrolled in the study, then blood sample were collected and tested for confirmation of the disease.[4] Patients were screened by their physician for the clinical symptom of typhoid fever which is fever of 2 or more days before admission accompanied by other clinical symptoms of typhoid fever in the absence of any other known febrile illnesses. Febrile patients whose presumptive clinical diagnosis were typhoid fever and sent to the laboratory by their physician for Widal test were included in the study. However, those febrile patients who had received antibiotic treatment for their symptom within two weeks before coming to the hospital and those who diagnosed for other known febrile illness were not included in this study. [5] By using these inclusion and exclusion criteria about 62 suspected febrile patients were recruited for this study then data and blood sample were collected from these 84 patients.
Sub culturing and biochemical identification
After 24 hours incubation sub-culturing was performed from the Typtic Soya broth on XLD agar (OXOID, England). After overnight incubation positive cultures were proceed further while Negative broth cultures were incubated for seven days and sub cultured before reported negative. Suspected colonies obtained on the above media were screened by biochemical tests using urease test (Himedia ltd. India) and lysine decarboxylation (LDC) [Difco™] test. [6]
Widal test
Qualitative slide agglutination and semi quantitative tube agglutination (titration) were performed using febrile antigen kits of Salmonella typhi (Chromatest Febrile Antigens kits, Linear chemicals, Barcelona, Spain). The slide agglutination test is used as a screening test for the presence of anti TO and anti TH antibodies in the patient’s serum. For the slide agglutination test a drop of Salmonella typhi O and H antigens are added on a drop of serum on card and rotated at 100 rpm for one minute and reported as reactive or non reactive. For those slide agglutinations whose results are reactive and weakly reactive titer was determined. In the tube agglutination test (titration), serum sample was serially diluted by using fresh 0.95% saline preparation from 1:20 to 1:640 for anti TO and anti TH separately in 12 test tubes. Then a drop of O antigens and H antigens are added in the test tubes, equal amount in all. Based on the manufacturer manual, an antibody titer of 1:80 and higher for anti TO and 1:160 and higher for anti TH antibodies were taken as a cut of value to indicate recent infection of typhoid fever.[7]
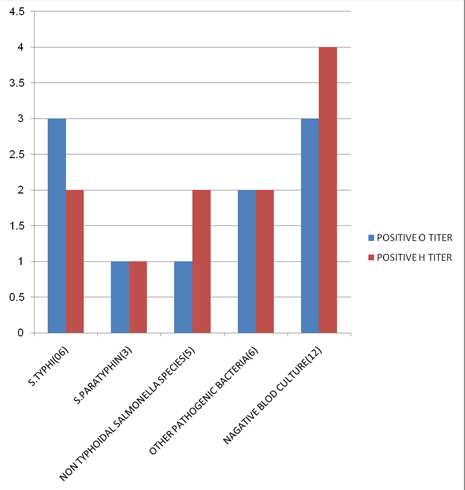
|
S. No. |
Bacteria |
Number of isolation |
|
01 |
S.typhi |
06 |
|
02 |
S. Paratyphi |
03 |
|
03 |
Non typhoid salmonella |
05 |
|
04 |
Other bacteria |
06 |
|
05 |
Negative blood culture |
12 |
Slide Test
Place one drop of positive control on one reaction circles of the slide
Pipette one drop of Isotonic saline on the next reaction cirlcle (-ve Control
Pipette one drop of the patient serum tobe tested onto the remaining four reaction circles.
Add one drop of Widal TEST antigen suspension ‘H’ to the first two reaction circles. (PC & NC).
Add one drop each of ‘O’, ‘H’, ‘AH’ and ‘BH’ antigens to the remaining four reaction circles.
Contents of each circle uniformly over the entire circle with separate mixing sticks
Rock the slide, gently back and forth and observe for agglutination macroscopically within one minute.
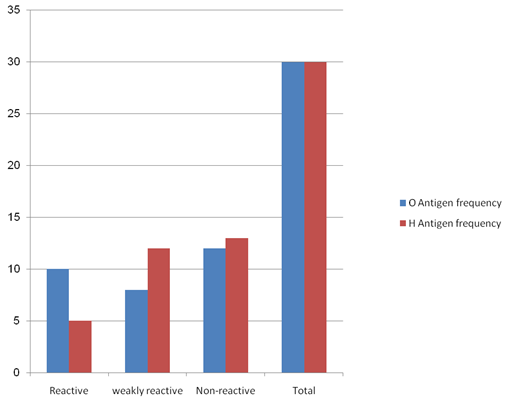
|
S.No. |
Reaction result |
O antigen frequency |
H antigen frequency |
|
01 |
Reactive |
10 |
05 |
|
02 |
Weakly Reactive |
08 |
12 |
|
03 |
Non Reactive |
12 |
13 |
|
04 |
Total |
30 |
30 |
Results
Comparisons of results
A comparative study of Widal test with blood culture in the diagnosis of typhoid fever in febrile patients
Observation and result
No agglutination = Negative
Result reported as 'titres' : Highest dilution where agglutination is
Seen
If agglutination appear after 15 seconds = (1:640
If agglutination appear after 30 seconds = (1:320
If agglutination appear after 1 min = (1:160
If agglutination appear after 1 30 min = (1:80
This test is a screening test only for the detection of Widal agglutinins
If result is positive it must be confirmed by other serological tests for Widal.
Discussion
The sensitivity and specificity of Widal titer of anti TO 1:80 and higher in this study were about 71.4% and 74.1% respectively and 28.6% and 86.3% for ant TH titer of 1:160 and higher. The overall sensitivity of titer positive Widal test was about 71.4%, similar with anti TO titer because there was no only anti TH titer positive culture proven typhoid fever identified.[8] This is similar with the study conducted in the endemic area of Vietnam by Olsen et al. Another study done in Kenya has shown that Widal testing done on acute phase serum of patients suspected to has typhoid fever had limited diagnostic capability given its low sensitivity in which among all typhoid cases only 26% had diagnostic titer while had O and H titer less than 1:40 With the cut off value of anti TO ≥1:80 and anti TH ≥ 1:160 Widal titer in this study, Widal test had relatively good NPV but PPV was very low Positive predictive value is more important than other measure of clinical diagnostic methods because it gives the proportion of patients with positive test results that are correctly diagnosed but it is highly affected by a prevalence of the disease.[9], [10]
Acknowledgement
I would also like to thank Dr. D. Vijay Kumar, H.O.D.for his support and encouragement.
Conflicts of Interest
All contributing authors declare no conflicts of interest.
Source of Funding
None.
References
- G Andualem, T Abebe, N Kebede, S Gebre-Selassie, A Mihret, H Alemayehu. A comparative study of Widal test with blood culture in the diagnosis of typhoid fever in febrile patients. BMC Res Notes 2014. [Google Scholar] [Crossref]
- V Gopalakrishnan, W Y Sekhar, E H Soo, R A Vinsent, S Devi. Typhoid fever in Kuala Lumpur and a comparative evaluation of two commercial diagnostic kits for the detection of antibodies to salmonella typhi. Singapore Med J 2002. [Google Scholar]
- EF Nsutebu, P Martins, D Adiogo. Short communication: Prevalence of typhoid fever in febrile patients with symptoms clinically compatible with typhoid fever in Cameroon. Trop Med Int Health 2003. [Google Scholar] [Crossref]
- CA Onyekewere. Typhoid fever: misdiagnosis or over diagnosis. Niger Med Pract 2007. [Google Scholar] [Crossref]
- S Hosoglu, J Wain. The laboratory diagnosis of enteric fever. J Infect Dev Ctries 2008. [Google Scholar] [Crossref]
- CM Parry, NTT Hoa, TS Diep, J Wain, NT Chinh, H Vinh. Value of a Single-Tube Widal Test in Diagnosis of Typhoid Fever in Vietnam. J Clin Microbiol 1999. [Google Scholar] [Crossref]
- J Wain, TS Diep, PVB Bay, AL Walsh, H Vinh, NM Duong. Specimens and culture media for the laboratory diagnosis of typhoid fever. J Infect Dev Ctries 2008. [Google Scholar] [Crossref]
- B Ley, G Mtove, K Thriemer, K Thriemer, B Amos, L V Seidlein. Evaluation of the Widal tube agglutination test for the diagnosis of typhoid fever among children admitted to a rural hospital in Tanzania and a comparison with previous studies. BMC Infect Dis 2011. [Google Scholar]
- G Beyene, D Asrat, Y Mengistu, A Aseffa, J Wain. Typhoid fever in Ethiopia. J Infect Dev 2008. [Google Scholar] [Crossref]
- LA Olopoenia. Classic methods revisited: Widal agglutination test - 100 years later: still plagued by controversy. Postgrad Med J 2000. [Google Scholar] [Crossref]
