- Visibility 55 Views
- Downloads 4 Downloads
- DOI 10.18231/j.ijmmtd.2024.058
-
CrossMark
- Citation
Bacteriological profile of breast abscess and its antimicrobial susceptibility at tertiary care hospital
Introduction
A breast abscess is inflammation of the breast characterized by localized infection and the formation of a contained pocket of pus.[1], [2] It is secondary to an untreated mastitis or mastitis that does not respond to the initial antibiotic regimen.[3]
Types of breast abscess
Lactational breast abscess
The majority of breast abscesses arise as a complication of lactational mastitis. [4] The occurrence rates range from approximately 0.4% to 11% in both developed and developing countries. [2], [3], [4], [5] This condition often results in significant morbidity among lactating women, frequently necessitating cessation of breastfeeding. [1] Risk factors contributing to the formation of lactational breast abscess include first pregnancy at maternal age over 30 years, pregnancy exceeding 41 weeks of gestation, primigravida, cracked nipples, encountering breastfeeding challenges during hospitalization, being a working mother, and mastitis. [1], [4]
Non-lactational breast abscess
These infections commonly involve a combination of aerobic and anaerobic skin flora [6]. Patients with non-lactational abscesses, particularly diabetics and smokers, are prone to recurrent infections. [4], [6]
Pathology and microbiology
The primary organism responsible for breast abscesses is often Staphylococcus aureus, with many isolated strains showing resistance to penicillin.[1], [4], [5], [6], [7], [8] In recent years, methicillin-resistant Staphylococcus aureus (MRSA) detection rates in breast milk and pus from lactating patients have been increasing gradually. [1], [5] Occasionally, a variety of other organisms may also be encountered. Entry of the organism typically occurs through a cracked nipple. [4], [6] Milk serves as an optimal culture medium and stasis of milk allows rapid spread of infection within the breast tissue and through milk ducts if untreated. [2], [4] Occasionally infection is hematogenous as seen in cases of tubercular breast infection.[1], [9]
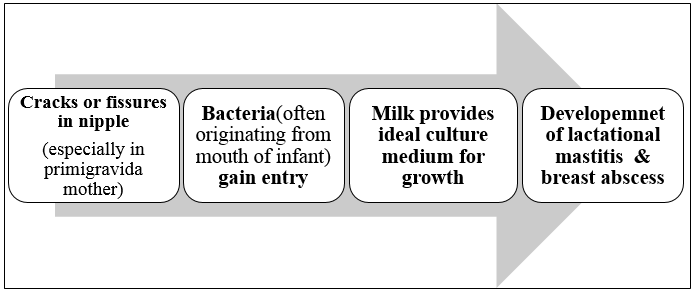
Patients commonly present with symptoms such as fever, chills, and malaise. The abscess manifests as a well-defined, tender lump with fluctuation, accompanied by redness and swelling of the affected breast. [2], [4]
Treatment options for abscesses commonly include antibiotics, ultrasound-guided needle aspiration or incision and drainage (I&D), although the optimal approach remains debated. [1] Gaining knowledge about the pathogens responsible for breast abscesses and their resistance patterns to different antibiotics can enhance the effectiveness of treatments for acute bacterial mastitis and breast abscesses by guiding surgeons in choosing the appropriate empirical therapy. Therefore, the present study was conducted to address microbiological profile of breast abscess and elucidates antimicrobial susceptibility of isolates.
Materials and Methods
Inclusion criteria
Pus aspirates received in the department of microbiology from patients presenting with breast abscess irrespective of their lactational status.
All age groups were included.
Exclusion criteria
Patients for whom adequate clinical data (age, probable diagnosis, lactational status) was not available were excluded from study.
Patients diagnosed with other benign conditions of breast and breast cancer were not included in the studies.
Sample collection
This study was a retrospective study conducted at tertiary care hospital, Jamnagar, Gujarat from July 2023 to June 2024.Total 100 samples from suspected breast abscess cases were collected with all aseptic precautions. Patients of all age groups were included. Data like age, sex, ward, indoor or outdoor cases, presence of Diabetes mellitus or other comorbid conditions and history of lactation of the patients were obtained. All pus aspirate samples were sent immediately to the department of microbiology and were processed according to standard protocol.
Sample processing
Day-1
Direct smear examination – smear was prepared from all samples and gram staining was done to detect presence of any pathogenic microorganisms.
Culture: All the samples were inoculated on Nutrient agar, Blood agar & MacConkey agar. Culture plates were incubated at 37°C overnight under aerobic conditions.
Day-2
Inoculated plates were checked for the presence of growth. Morphology of colonies were noted. Gram staining of colonies was done to differentiate between gram positive and gram-negative organisms. According to results of gram staining battery of biochemical reactions were selected.
All identified isolates were tested for susceptibility against the commercially available antibiotic discs by modified Kirby-Bauer disc diffusion method on Mueller–Hinton agar (MHA). 0.5 McFarland bacterial suspensions were prepared from a single isolated colony. Lawn cultures of this bacterial suspension were performed over the MHA plate. Subsequently, antibiotic discs were placed on agar surface using sterile forceps. Finally, the plates were incubated aerobically at 37 °C for 24 hrs. The antibiotics to be tested were selected according to latest CLSI guidelines [10].
Day-3
Results of all biochemical tests were noted. Zones of antibiotic sensitivity testing were compared with the reference zones in CLSI guidelines [10].
Results
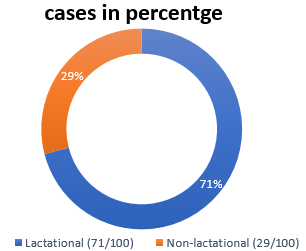
A total 100 pus aspirates from patients with suspected breast abscesses were received. All patients were female. From 100, 71 were lactating and 29 were non-lactating [[Figure 2]]. Age of presentation ranged from 19 to 75 years. Mean age was 31± 5 years.[[Table 1]] There was no any significant difference between numbers of right and left breast abscess, there was one case of bilateral breast abscess.
|
Age |
Frequency (n=100) |
Percentage (%) |
|
< 20 years |
7 |
7% |
|
21-30 years |
55 |
55% |
|
31-40 years |
18 |
18% |
|
41-50 years |
12 |
12% |
|
51-60 years |
2 |
2% |
|
>60 years |
6 |
6% |
Out of 100 pus samples 79 samples were culture positive, 21 samples were culture negative. From all positive samples (n=79) S. aureus was most common isolate, which was 66 (83.5%). Out of all S. aureus isolates, 39 (59%) were MSSA and 27 (41%) were MRSA. Identification of MRSA was done by testing susceptibility to cefoxitin. Other gram-positive organism isolated was Enterococcus species 1(1.3%). Gram negative organisms isolated were Klebsiella pneumoniae 3(3.8%), Pseudomonas aeruginosa 3(3.8%), Acinetobacter species 2(2.5%), Proteus mirabilis 2(2.5%), Escherichia coli 1(1.3%), Morganella morganii 1(1.3%) as shown in [Table 2].
|
Organism Isolated |
Total (n=100) |
Proportion (n=100) |
Frequency (n=79) |
|
Gram positive bacteria |
|
|
|
|
Staphylococcus aureus |
66 |
66 |
83.5% |
|
MSSA |
39 |
- |
- |
|
MRSA |
27 |
- |
- |
|
Enterococcus species |
1 |
1 |
1.3% |
|
Subtotal |
67 |
67 |
84.8% |
|
Gram negative bacteria |
|
|
|
|
Klebsiella pneumoniae |
3 |
3 |
3.8% |
|
Pseudomonas aeruginosa |
3 |
3 |
3.8% |
|
Acinetobacter species |
2 |
2 |
2.5% |
|
Proteus mirabilis |
2 |
2 |
2.5% |
|
Escherichia coli |
1 |
1 |
1.3% |
|
Morganella morganii |
1 |
1 |
1.3% |
|
Subtotal |
12 |
12 |
15.2% |
|
Negative culture |
21 |
21 |
- |
|
Total |
100 |
100 |
- |
|
Proportion = % of isolates to the total number (n=100) frequency = % of the organisms to the number of total culture -positive patients (n=79) |
The Susceptibility results for MSSA and MRSA are shown in [Table 3]. D-test was performed to look for inducible clindamycin resistance in staphylococcal isolates. None of the MSSA were D-test positive, however, two MRSA isolates were positive. Beside S.aureus other gram-positive organism isolated was Enterococcus species (n=1), which was sensitive to drugs like linezolid, streptomycin and high level gentamicin. However, it was resistant to ampicillin. The antimicrobial susceptibility results of gram-negative organisms isolated are shown in [Table 4]. All gram negative isolates showed 100% sensitivity cotrimoxazole, tetracyclines and carbapenem group of antibiotics.
All patients went under incision and drainage or USG guided fine needle aspiration followed by administration of appropriate empirical antibiotic. Antibiotics administered were modified after antimicrobial susceptibility test report.
|
Drugs |
S.aureus (n=66) isolates susceptibility |
|||
|
MSSA |
*MRSA |
|||
|
n=39 |
% |
n=27 |
% |
|
|
Cefoxitin (30 μg) |
39 |
100 |
0 |
0 |
|
Amoxicillin-clavulanic acid (20/10μg) |
39 |
100 |
- |
- |
|
Erythromycin (15 μg) |
31 |
79.5 |
9 |
33.3 |
|
Clindamycin (2 μg) |
39 |
100 |
9 |
33.3 |
|
Tetracycline (30 μg) |
39 |
100 |
27 |
100 |
|
Doxycycline (30 μg) |
39 |
100 |
27 |
100 |
|
Minocycline (30 μg) |
39 |
100 |
27 |
100 |
|
Cotrimoxazole [Trimethoprim-sulfamethoxazole 1.25/23.75 μg ] |
33 |
84.6 |
18 |
66.7 |
|
Linezolid (30 μg) |
39 |
100 |
27 |
100 |
|
Ciprofloxacin (5 μg) |
29 |
74.4 |
14 |
51.9 |
|
Levofloxacin (5 μg) |
39 |
100 |
18 |
66.7 |
|
Gentamicin (10 μg) |
36 |
92.3 |
27 |
100 |
|
*Identification of MRSA was done by testing susceptibility to cefoxitin |
|
Antimicrobial agent |
P.aeruginosa (n=3) |
Acinetobacter spp. (n=2) |
P.mirabilis (n=2) |
K.pneumoniae (n=3) |
E.coli (n=1) |
M.morganii (n=1) |
|
Ampicillin(10μg) |
IR |
IR |
1(50) |
IR |
1(100) |
IR |
|
Cefuroxime(30μg) |
- |
- |
1(50) |
2(66.7) |
1(100) |
IR |
|
Ceftriaxone(30μg) |
IR |
1(50) |
2(100) |
2(66.7) |
1(100) |
1(100) |
|
Cefotaxime(30μg) |
IR |
1(50) |
2(100) |
2(66.7) |
1(100) |
1(100) |
|
Ceftazidime(30μg) |
2(66.7) |
1(50) |
2(100) |
2(66.7) |
1(100) |
1(100) |
|
Cefepime (30μg) |
2(66.7) |
1(50) |
2(100) |
3(100) |
1(100) |
1(100) |
|
Amoxicillin-clavulanic acid (20/10μg) |
IR |
IR |
2(100) |
2(66.7) |
1(100) |
IR |
|
Ampicillin-sulbactam (10/10μg) |
IR |
1(50) |
2(100) |
2(66.7) |
1(100) |
1(100) |
|
Ceftazidime-avibactam (30/20μg) |
2(66.7) |
- |
2(100) |
2(66.7) |
1(100) |
1(100) |
|
Piperacillin-tazobactam (100/10μg) |
2(66.7) |
1(50) |
2(100) |
2(66.7) |
1(100) |
1(100) |
|
Imipenem(10μg) |
3(100) |
- |
2(100) |
3(100) |
1(100) |
1(100) |
|
Meropenem(10μg) |
3(100) |
2(100) |
2(100) |
3(100) |
1(100) |
1(100) |
|
Ertapenem(10μg) |
IR |
IR |
2(100) |
3(100) |
1(100) |
1(100) |
|
Aztreonam(30μg) |
2(66.7) |
IR |
2(100) |
3(100) |
1(100) |
1(100) |
|
Gentamicin(10μg) |
- |
1(50) |
2(100) |
2(66.7) |
1(100) |
1(100) |
|
Amikacin(30μg) |
- |
1(50) |
2(100) |
2(66.7) |
1(100) |
1(100) |
|
Ciprofloxacin(5μg) |
2(66.7) |
1(50) |
2(100) |
3(100) |
1(100) |
1(100) |
|
Levofloxacin(5μg) |
2(66.7) |
2(100) |
2(100) |
3(100) |
1(100) |
1(100) |
|
Cotrimoxazole [Trimethoprim-sulfamethoxazole 1.25/23.75μg ] |
IR |
2(100) |
2(100) |
3(100) |
1(100) |
1(100) |
|
Tetracycline(30μg) |
IR |
- |
IR |
3(100) |
1(100) |
1(100) |
|
Doxycycline(30μg) |
IR |
2(100) |
IR |
3(100) |
1(100) |
1(100) |
|
Minocycline(30μg) |
IR |
2(100) |
IR |
3(100) |
1(100) |
1(100) |
|
Data presented as n (% susceptible) IR-intrinsically resistant |
|
Discussion
The breast consists of numerous lobules, which are especially abundant in young women. Each lobule drains into a lactiferous duct and lactiferous sinus, which then release milk onto the nipple's surface. [11], [12] During a woman's life, the breast undergoes physiological changes during menstruation, pregnancy, childbirth and menopause. [3] During pregnancy, the ductal system expands, and there is proliferation and elongation of lactiferous ducts. The lactiferous sinuses serve as reservoirs for milk during lactation. [12]
Bacterial mastitis is most often associated with lactation.[11] Infection in the breast can occur through lymphatic or blood route, direct introduction of pathogens (via cracked nipples), or through spread from nearby structures. Typically, the infection starts in the lactiferous ducts, which can become obstructed by skin debris, leading to milk stasis. Since milk serves as a perfect environment for bacterial growth, it facilitates the spread of bacteria. This results in acute inflammation and allows the infection to spread quickly through the surrounding tissue. [3], [11]
In present study, majority cases were lactating women (71%), similar to study of Jena P et al (67.7%)[3] and Ullah K et al[13] (70.1%). In the study of Antonello VS et al[14] only 31.5% cases lactation was found as risk factor, this might be due to highest obesity prevalence and smoking rates seen in developed countries like Brazil.
The age at presentation ranged from 19 to 75 years and the mean age was 31± 5 years. Similar results for mean age were seen in study of Li, Y. et al (30.6 years) [5], Ullah K et al[13] (30 years with SD ± 1.58) and Li D et al[15] (29 years). The high occurrence of lactational breast abscesses in young mothers can be attributed to poor breastfeeding techniques, poor hygiene, and lack of awareness and education. Other risk factors founded in present study were diabetes mellitus, smoking and obesity.
Although the study identified several types of microorganisms, S. aureus (83.5%) was the most common isolate. The S.aureus was the most common isolate in all the studies. The staphylococcal isolation rate and MRSA rate of different studies are described in [Table 5].
|
Author/year |
Most Common isolate (%) |
MRSA rate (%) * |
|
Moazzez A et al,2007 [16] |
S.aureus (32%) |
58% |
|
Ramakrishnan R et al,2017 [7] |
S.aureus (84%) |
- |
|
Jena P et al.,2019 [3] |
S.aureus (83.3%) |
49% |
|
Pileri, P et al.,2022 [1] |
S.aureus (90%) |
44.8% |
|
Li D et al.,2022 [15] |
S.aureus (63%) in abscess group |
42% |
|
Antonello VS et al,2024 [14] |
S.aureus (70.6%) |
26% |
|
Present study 2024 |
S.aureus (83.5%) |
41% |
|
* Percentage of MRSA out of total S.aureus S.aureus- Staphylococcus aureus; MRSA-Methicillin resistant S.aureus |
The S.aureus isolation rate of present study(83.5%) was similar to the study of Jena P et al (83.3%),[3] Li, Y. et al (82.3%)[5] and Ramakrishnan R et al[7] (84%). It has been documented that full-term infants can be colonized by Staphylococci in the first few days after birth, with potential sources including nursing personnel or other infants in the hospital.[3] Once inside the ampulla of the duct, staphylococci induce milk clotting, allowing the bacteria to proliferate within the clot. Initially, the affected breast, or often just a portion of it, exhibits signs of acute inflammation as a generalized cellulitis, which eventually progresses to form an abscess.[11] Abscesses commonly develop in S.aureus skin and soft tissue infections as a way to localize the infection. Neutrophils, the main immune cells responsible for fighting S.aureus, release antimicrobial agents that kill bacteria but may also cause tissue damage and contribute to abscess formation. In response, S.aureus secretes molecules that encourage abscess formation by attracting neutrophils, breaking down host cells, and aiding in creation of a fibrin capsule around the abscess.[17]
Out of total S.aureus isolates (n=66), 39 (59%) were MSSA and 27 (41%) were MRSA. The MRSA rate of present study (41%) was similar to studies like Jena P et al.[3] (49%), Pileri P et al[1] (44.8%) and Li D et al.[15] (42%). Although the specific cause of the MRSA increase is unknown, one possible explanation is that these lactating mothers might have encountered MRSA during their hospital stay in their peripartum period, leading to its emergence. [18]
AMR Surveillance Network (ICMR) annual report of 2022 showed proportion of MRSA was 44.5% in the year 2022, which was slightly higher than the rate reported in 2021 (42.6%). According to that report overall MRSA rates have increased each year from 2017 to 2022 (32.9% to 45.5%).[19] MRSA signifies resistance to all beta-lactam antibiotics and is mediated by mecA and mecC gene coding for penicillin binding protein 2a.[3] Recently plasmid mediated mecB and mecD genes have also been reported in S.aureus. [20], [21] Comparison of susceptibility rates of different antimicrobial agents for MSSA and MRSA are given in [Table 6], [Table 7] respectively.
|
Antimicrobial agent |
Susceptibility rates (S%) for MSSA |
|
|
Present study (S%) |
Other studies (S%) |
|
|
Amoxicillin-clavulanic acid (20/10μg) |
100% |
Khalifa Al et al. [22] (97%) |
|
Clindamycin(2μg) |
100% |
Lodhi N et al.[18] (100%) |
|
Jena P et al.[3] (95%) |
||
|
Cotrimoxazole [Trimethoprim-sulfamethoxazole 1.25/23.75μg ] |
84.6% |
Ullah K et al. [13] (89.9%) |
|
Erythromycin(15μg) |
79.5% |
INSAR Group 2013 et al.[23] (73.7%) |
|
Gentamicin(10μg) |
92.3% |
Li D et al.[15] (92.5%) |
|
Antonello VS et al.[14] (98.9%) |
||
|
Linezolid(30μg) |
100% |
Antonello VS et al.[14] (100%) |
|
INSAR Group 2013 et al.[23] (100%) |
||
|
Tetracycline(30μg) |
100% |
Li D et al.[15] (94%) |
|
Antimicrobial agent |
Susceptibility rates (S%) for MRSA |
|
|
Present study (S%) |
Other studies (S%) |
|
|
Clindamycin (2μg) |
33.3% |
INSAR Group 2013 et al.[23] (53.4%) |
|
Cotrimoxazole [Trimethoprim-sulfamethoxazole 1.25/23.75μg ] |
66.7% |
Ullah K et al. [13] (68.4%) |
|
ICMR AMRSN Annual report of 2022 et al.[19] (69.8%) |
||
|
Erythromycin (15μg) |
33.3% |
INSAR Group 2013 et al.[23] (29.2%) |
|
Gentamicin (10μg) |
100% |
Li Y et al.[5] (96.2%) |
|
Li D et al.[15] (97.1%) |
||
|
Linezolid (30μg) |
100% |
AMRSN Annual report of 2022 et al.[19] (97.3%) |
|
Tetracycline (30μg) |
100% |
Li D et al.[15] (88.6%) |
General strategies for managing a breast abscess involve using analgesics, providing breast support, breast emptying and continuing breastfeeding, and administering anti-staphylococcal antibiotics. Specific treatments include ultrasound guided needle aspiration or catheter drainage, surgical incision and drainage. [4]
While choosing empirical therapy, it is important to consider the antibiotic susceptibility of isolates in the specific geographical region and the local antibiogram. The pus collection drained from abscess should be cultured. [3], [24] The majority of mastitis cases that develop into a breast abscess are caused by S.aureus. [4] For treatment of mastitis cloxacillin and its congeners are preferred as they treat cellulitis well. For treatment of breast abscess erythromycin is considered as being alkaline it is well concentrated and remains active in breast milk. In case of penicillin allergy clindamycin can be used. [4] In centres where MRSA rates are high, vancomycin or linezolid may be used as empirical therapy with de-escalation if required.[19] It is recommended that duration of antibiotic therapy should be 10 days. [4]
For empirical therapy of skin and soft tissue infections caused by staphylococcus, drug of choice for methicillin sensitive isolates (MSSA) is oral dicloxacillin 500 mg orally 4 times daily/cephalexin 500 mg orally 4 times daily/ cefadroxil 1gm every 12 hourly. Alternative agents which can be used for both MSSA and MRSA are minocycline/doxycycline (100 mg orally twice daily), TMP-SMX (cotrimoxazole - 1 or 2 double-strength tablets orally twice daily), clindamycin (300–450 mg orally 3 times daily), and linezolid (600 mg orally twice daily). [3], [24]
Antibiotics alone are seldom effective in curing breast abscesses. Typically, abscesses need to be drained as well as treated with antibiotics to fully resolve. [3], [7], [9] Some centers use needle aspiration combined with appropriate antibiotic treatment since this method avoids the need for postoperative dressings, results in minimal post-operative discomfort hence breastfeeding can be continued, has fewer complications, and is more cosmetically favourable. However, the disadvantage of this procedure is that it may require multiple aspirations if the abscess is not fully drained or if it has multiple compartments. Incision & drainage is considered as first line of management in case of large abscesses (>5cm), multiple or prolonged abscesses. [2], [3]
In our institute, surgical management of breast abscess is decided according to size of abscess. Small abscesses are managed by needle aspiration and for large abscesses incision and drainage is preferred. Irrespective of size of abscess, empirical antibiotics are prescribed to all patients and later modified according to antibiotic sensitivity test reports. In the majority of cases, Augmentin (Amoxicillin-Clavulanate) was first administered as empirical therapy. According to results of present study Augmentin showed 100% sensitivity in MSSA. Use of Augmentin as initial empirical therapy was supported in the study of Ramakrishnan R et al. [7] Erythromycin or Clindamycin can be considered as alternative agents. For breast abscesses caused by MRSA oral antibiotic agents like trimethoprim/sulfamethoxazole, doxycycline or clindamycin can be used. Mothers should avoid breastfeeding while on trimethoprim/sulfamethoxazole if their infant is under 2 months old. Additionally, breastfeeding should be discontinued if the mother is on doxycycline. For more severe cases or in hospitalized patients with suspected hospital-acquired MRSA, vancomycin (15 mg/kg intravenously every 12 hours) can be used. [9]
Toxic shock syndrome (TSS) due to a breast abscess in adults is rarely documented. A 2020 case report from Nepal described a 19-year-old woman who developed toxic shock syndrome in whom source of infection was abscess in her left breast and the causative organism was MRSA. They confirmed the case by using CDC criteria. Treatment for this condition should involve a penicillinase-resistant penicillin, cephalosporin, or vancomycin (especially in areas where MRSA is common), along with either clindamycin or linezolid. In this case, treatment included incision and drainage (I&D) of the breast abscess, along with vancomycin and clindamycin. [25]
Clindamycin is favoured for treating MRSA not only because of its bactericidal effect but also because it inhibits toxin production. [3] The present study showed that clindamycin is susceptible in 33.3% of MRSA isolates. The lower susceptibility to Clindamycin is might be due to inducible or constitutive MLSB resistance. The rising prevalence of methicillin resistance in Staphylococci is a significant issue, prompting renewed interest in Macrolide Lincosamide-Streptogramin B (MLSB) antibiotics for treating S. aureus infections. Clindamycin is particularly preferred due to its excellent pharmacokinetic properties. However, the widespread use of MLSB antibiotics has also resulted in a growing number of staphylococcal strains developing resistance to these drugs [26]. Clindamycin is associated with either inducible or constitutive resistance in roughly 50% of MRSA isolates. [3]
In the present study all gram-negative organisms showed good sensitivity towards levofloxacin and carbapenem group of antibiotics. Organisms like K.pneumoniae and Acinetobacter showed decreased sensitivity to third generation cephalosporins and beta-lactam combination agents. If gram-negative bacilli are identified, a quinolone such as levofloxacin can be used if the patient is not breastfeeding. Alternatively, a third-generation cephalosporin, like ceftriaxone or cefotaxime, can be used to treat infections caused by gram-negative bacilli. [9]
Timely diagnosis and proper treatment are vital because mastitis and abscesses are significant factors contributing to early weaning, resulting in the loss of breastfeeding benefits for both mother and child. Moreover, inadequately treated abscesses can potentially lead to sepsis and in rare cases, be fatal. [1]
Conclusion
As it is evident from the present study that breast abscess may present in women at any age group. Although variety of pathogens got isolated, S.aureus was the predominant organism, isolated in both lactational and non-lactational group. It is very crucial to give proper antimicrobial treatment in initial phase of mastitis as it can prevent progression to abscess formation. Surgical management along with use of antibiotics is necessary for management of breast abscess. For our institute recommended drugs for an initial empirical therapy are amoxycillin-clavulanic acid, erythromycin and clindamycin. Later on, the therapy should be modified according to antimicrobial sensitivity test report. The maximum occurrence of breast abscess was seen in lactating women (71%). Non-lactational causes identified were diabetes mellitus, smoking and obesity. Pregnant and lactating mothers should be trained and counselled about breast feeding and hygiene of breast. Adherence to good infection control practices in maternity wards can help in decreasing chances of acquiring infection during hospital stay. Although cracked nipples are cause of breast abscess, breast feeding should not be stopped as milk stasis can further aggravate the condition.
Limitations
In present study samples were not analysed for the presence of anaerobic organisms and Mycobacteria.
Vancomycin susceptibility was not assessed because the Vancomycin broth dilution method, as outlined by standard CLSI guidelines, was not available.
Ethical Approval
The Institutional Ethics Committee of Shri M.P. Shah Government Medical College and Guru Gobind Singh Hospital in Jamnagar approved this study with reference number 288/03/2023 on February 22, 2024.
Conflict of Interests
The authors declare no conflicts of interest in this work.
Source of Funding
None.
References
- P Pileri, A Sartani, MI Mazzocco, S Giani, S Rimoldi, G Pietropaolo. Management of Breast Abscess during Breastfeeding. Int J Environ Res Public Health 2022. [Google Scholar] [Crossref]
- F Zhou, Z Li, L Liu, F Wang, L Yu, Y Xiang. The effectiveness of needle aspiration versus traditional incision and drainage in the treatment of breast abscess: a meta-analysis. Ann Med 2023. [Google Scholar] [Crossref]
- P Jena, S Duggal, R Gur, A Kumar, T Bharara, R Dewan. Staphylococcus aureus in breast abscess-major culprit besides others. Indian J Med Sci 2019. [Google Scholar]
- K Kataria, A Srivastava, A Dhar. Management of Lactational Mastitis and Breast Abscesses: Review of Current Knowledge and Practice. Indian J Surg 2012. [Google Scholar]
- Y Li, XJ Ma, XP He. Clinical characteristics of lactational breast abscess caused by methicillin-resistant Staphylococcus aureus: hospital-based study in China. Int Breastfeed J 2021. [Google Scholar] [Crossref]
- JCM Townsend, RD Beauchamp, BM Evers, KL Mattox. . Sabiston Textbook of Surgery 2016. [Google Scholar]
- R Ramakrishnan, RV Trichur, S Murugesan, S Cattamanchi. Analysis of the microbial flora in breast abscess: a retrospective cohort study conducted in the emergency department. Int Surg J 2017. [Google Scholar]
- W Branch-Elliman, TH Golen, HS Gold, DS Yassa, LM Baldini, SB Wright. Risk Factors for Staphylococcus aureus Postpartum Breast Abscess. Clin Infect Dis 2012. [Google Scholar]
- E Boakes, A Woods, N Johnson, Kadoglou. Breast Infection: A Review of Diagnosis and Management Practices. Eur J Breast Health 2018. [Google Scholar]
- . CLSI M100 Performance Standards for Antimicrobial Susceptibility Testing, 34th Edition. 2024. [Google Scholar]
- N Williams, PR O’Connell, A McCaskie. . Bailey & Love’s Short Practice of Surgery 2018. [Google Scholar]
- A Alex, E Bhandary, KP McGuire. Anatomy and Physiology of the Breast during Pregnancy and Lactation. Adv Exp Med Biol 2020. [Google Scholar] [Crossref]
- KU Muslihuddin, N Ahmad, Z Jan, A Shoaib. Bacteriology and Antibiotic sensitivity of Breast Abscess. J Saidu Med Coll Swat 1921. [Google Scholar]
- VS Antonello, J Dallé, MF Jimenez, P Tramontini, AG Reginatto. Bacteriological characteristics of primary breast abscesses in patients from the community in the era of microbial resistance. Rev Bras Ginecol Obstet 2024. [Google Scholar] [Crossref]
- D Li, J Li, Y Yuan, J Zhou, Q Xiao, T Yang. Risk factors and prognosis of acute lactation mastitis developing into a breast abscess: A retrospective longitudinal study in China. PLoS ONE 2022. [Google Scholar] [Crossref]
- A Moazzez, RL Kelso, S Towfigh, H Sohn, TV Berne, RJ Mason. Breast abscess bacteriologic features in the era of community-acquired methicillin-resistant Staphylococcus aureus epidemics. Arch Surg 2007. [Google Scholar]
- SD Kobayashi, N Malachowa, FR DeLeo. Pathogenesis of Staphylococcus aureus Abscesses. Am J Pathol 2015. [Google Scholar]
- N Lodhi, N Khurshaidi, R Soomro, M Saleem, SSU Rahman, S Anwar. Is our choice of empirical antibiotics appropriate for patients with methicillin resistant Staphylococcus aureus in breast abscess?. Iran J Microbiol 2018. [Google Scholar]
- . ICMR- Antimicrobial Resistance Surveillance Network Annual report of January-2022 to December 2022. . [Google Scholar]
- K Becker, S Van Alen, EA Idelevich, N Schleimer, J Seggewiß, A Mellmann. Plasmid-Encoded Transferable mecB-Mediated Methicillin Resistance in Staphylococcus aureus. Emerg Infect Dis 2018. [Google Scholar]
- S Lakhundi, K Zhang. Methicillin-Resistant Staphylococcus aureus: Molecular Characterization, Evolution, and Epidemiology. Clin Microbiol Rev 2018. [Google Scholar] [Crossref]
- KA Benwan, AA Mulla, VO Rotimi, Rotimi. A Study of the Microbiology of Breast Abscess in a Teaching Hospital in Kuwait. Med Princ Pract 2011. [Google Scholar]
- . Indian Network for Surveillance of Antimicrobial Resistance (INSAR) group, India. Methicillin resistant Staphylococcus aureus (MRSA) in India: prevalence & susceptibility pattern. Indian J Med Res 2013. [Google Scholar]
- J Loscalzo, A Fauci, D Kasper, S Hauser, D Longo, JL Jameson. . Harrison’s Principle of Internal medicine 2022. [Google Scholar]
- K Pandit, S Khanal, P Adhikari, S Adhikari, SP Acharya. Staphylococcal toxic shock syndrome in a lactating mother with breast abscess: A case report. Ann Med Surg 2020. [Google Scholar] [Crossref]
- K Prabhu, S Rao, V Rao. Inducible Clindamycin Resistance in Staphylococcus aureus Isolated from Clinical Samples. J Lab Physicians 2011. [Google Scholar]
